1
/
of
5
www.ChineseStandard.us -- Field Test Asia Pte. Ltd.
GB 1903.33-2022 English PDF
GB 1903.33-2022 English PDF
Regular price
$110.00
Regular price
Sale price
$110.00
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
GB 1903.33-2022: National food safety standard - Food nutritional fortification substance - 5'-CMP
Delivery: 9 seconds. Download (& Email) true-PDF + Invoice.
Get Quotation: Click GB 1903.33-2022 (Self-service in 1-minute)
Historical versions (Master-website): GB 1903.33-2022
Preview True-PDF (Reload/Scroll-down if blank)
GB 1903.33-2022
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National food safety standard - Food nutritional fortification
substance - 5'-CMP
ISSUED ON: JUNE 30, 2022
IMPLEMENTED ON: DECEMBER 30, 2022
Issued by: National Health Commission of PRC;
State Administration for Market Regulation.
Table of Contents
1 Scope ... 3
2 Chemical name, molecular formula, structural formula, relative molecular mass ... 3
3 Technical requirements ... 3
Appendix A Inspection method ... 5
Appendix B Standard infrared spectrum of 5'-cytidine monophosphate ... 9
National food safety standard - Food nutritional fortification
substance - 5'-CMP
1 Scope
This standard applies to the food nutrition fortifier - 5'-cytidine monophosphate, which
is produced by using yeast ribonucleic acid (RNA) as raw material, through nuclease
enzymatic hydrolysis.
2 Chemical name, molecular formula, structural formula,
relative molecular mass
2.1 Chemical name
5'-cytidine monophosphate (5'-cytidylic acid, 5'-CMP)
2.2 Molecular formula
C9H14N3O8P
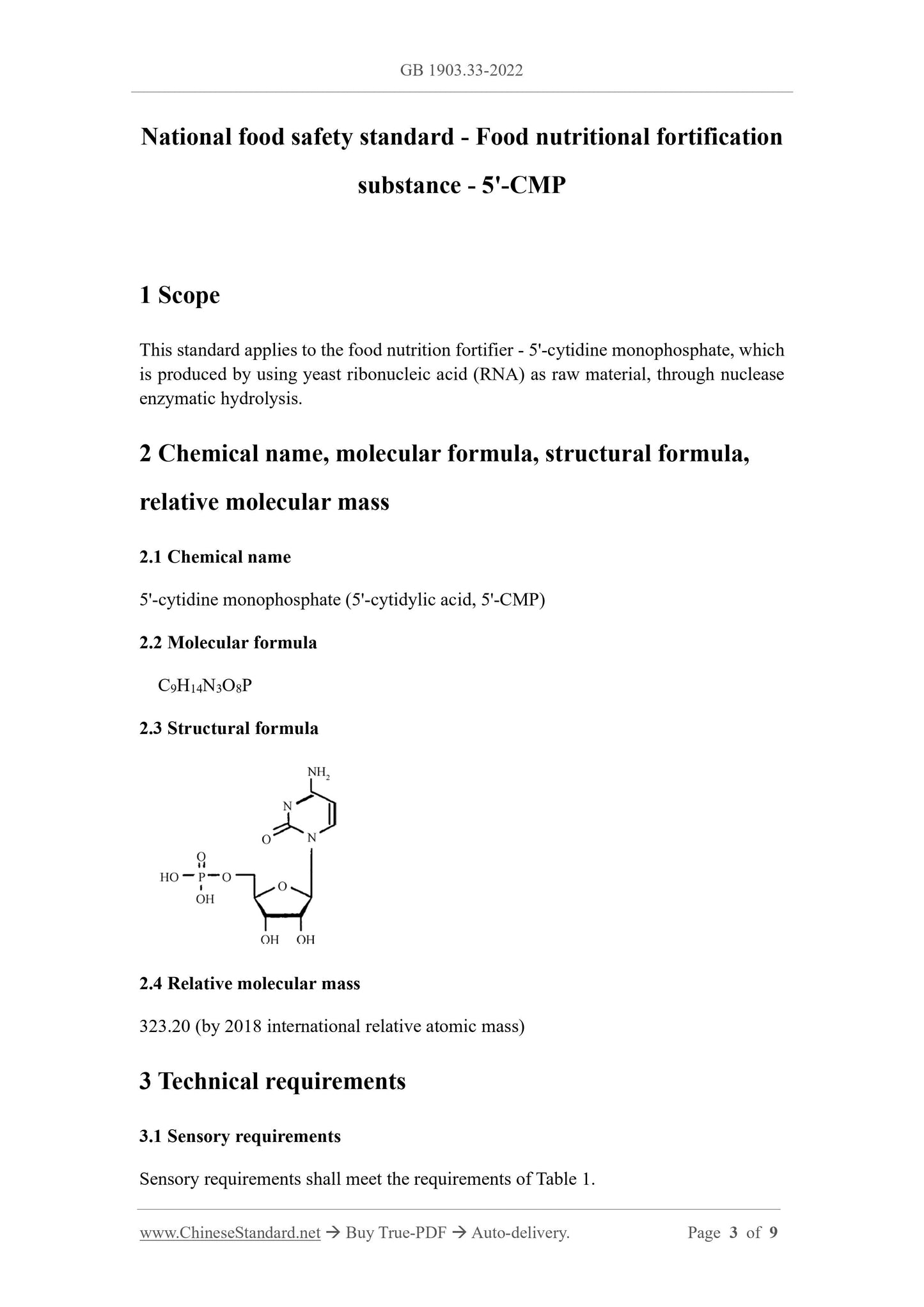
2.3 Structural formula
2.4 Relative molecular mass
323.20 (by 2018 international relative atomic mass)
3 Technical requirements
3.1 Sensory requirements
Sensory requirements shall meet the requirements of Table 1.
Appendix A
Inspection method
A.1 General provisions
The reagents and water used in this standard, unless other requirements are specified,
refer to the analytical reagents and the grade-3 water, which is specified in GB/T 6682.
The standard titration solution, standard solution for impurity determination,
preparations and products, which are used in the test, shall be prepared in accordance
with the provisions of GB/T 601, GB/T 602, GB/T 603, unless otherwise specified. The
solution used in the test refers to the aqueous solution, when the solvent is not specified.
A.2 Identification experiment
It is carried out, according to the method specified in GB/T 6040. The infrared spectrum
of the product shall be consistent with the infrared spectrum of the standard product
(see Appendix B, for the infrared spectrum of the standard product).
A.3 Determination of 5'-cytidine monophosphate (C9H14N3O8P) (on a dry basis)
A.3.1 Reagents and materials
A.3.1.1 Water: Grade-1 water in accordance with GB/T 6682-2008.
A.3.1.2 2 mol/L sodium hydroxide solution: Weigh 40 g of solid sodium hydroxide in
a 500 mL volumetric flask. Use water to dissolve it. Dilute it to the mark. Shake well.
A.3.1.3 0.05 mol/L potassium dihydrogen phosphate solution: Weigh 6.8045 g of
potassium dihydrogen phosphate (KH2PO4) into a 1 L volumetric flask. Use water to
dissolve it AND dilute it to the mark. Shake well. Use a 0.45 µm microporous
membrane for filtration. Perform ultrasonic degassing for 30 min, before use.
A.3.1.4 5'-cytidine monophosphate standard substance (purity ≥ 98%).
A.3.1.5 Standard stock solution of 5'-cytidine monophosphate: Accurately weigh 100.0
mg of 5'-cytidine monophosphate standard substance, in a 50 mL volumetric flask. Use
water to dissolve it. Add 1 ~ 2 drops of 2 mol/L sodium hydroxide solution, to help
dissolve. Make the volume to the mark. Mix well. Seal and store it in a 4 °C refrigerator,
to prepare for later use.
A.3.1.6 Standard use solution of 5'-cytidine monophosphate: Accurately pipette 1.0 mL
of 5'-cytidine monophosphate standard stock solution, into a 50 mL volumetric flask.
Use water to dilute it to the mark. Shake well. Use a 0.45 µm microporous membrane
for filtration, before injection.
A.3.2 Instruments and equipment
A.3.2.1 High performance liquid chromatography (ultraviolet detector).
A.3.2.2 Analytical balance (sensitivity 0.0001 g).
A.3.3 Reference liquid chromatography conditions
A.3.3.1 Chromatographic column: C18 (4.6 mm × 250 mm, 5 µm) or equivalent type
column.
A.3.3.2 Mobile phase: 0.05 mol/L KH2PO4 solution + methanol = 95 + 5.
A.3.3.3 Injection volume: 20 µL.
A.3.3.4 Flow rate: 0.8 mL/min.
A.3.3.5 UV detection wavelength: 254 nm.
A.3.3.6 Column oven temperature: 25 °C.
A.3.4 Preparation of specimen
Sample stock solution: Accurately weigh 100.0 mg of the sample into a 50 mL
volumetric flask. Use water to dissolve it. Add 1 ~ 2 drops of 2 mol/L sodium hydroxide
solution, to help dissolve. Make the volume to the mark. Mix well.
Sample use solution: Accurately pipette 1.0 mL of the sample stock solution, into a 50
mL volumetric flask. Use water to make the volume reach to the mark. Shake well. Use
a 0.45 μm microporous membrane for filtration, before injection.
A.3.5 Determination
According to the chromatographic conditions of A.3.3, inject the 5'-cytidine
monophosphate standard solution and the sample use solution, respectively. Based on
the retention time of the standard, determine the chromatographic peak of 5'-cytidine
monophosphate in the sample. According to the peak area of the sample, use the
external standard method, to calculate the content of 5'-cytidine monophosphate in the
sample.
A.3.6 Result calculation
The mass fraction w1 of 5'-cytidine monophosphate content (on a dry basis) is
calculated, according to formula (A.1).
Where:
GB 1903.33-2022
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National food safety standard - Food nutritional fortification
substance - 5'-CMP
ISSUED ON: JUNE 30, 2022
IMPLEMENTED ON: DECEMBER 30, 2022
Issued by: National Health Commission of PRC;
State Administration for Market Regulation.
Table of Contents
1 Scope ... 3
2 Chemical name, molecular formula, structural formula, relative molecular mass ... 3
3 Technical requirements ... 3
Appendix A Inspection method ... 5
Appendix B Standard infrared spectrum of 5'-cytidine monophosphate ... 9
National food safety standard - Food nutritional fortification
substance - 5'-CMP
1 Scope
This standard applies to the food nutrition fortifier - 5'-cytidine monophosphate, which
is produced by using yeast ribonucleic acid (RNA) as raw material, through nuclease
enzymatic hydrolysis.
2 Chemical name, molecular formula, structural formula,
relative molecular mass
2.1 Chemical name
5'-cytidine monophosphate (5'-cytidylic acid, 5'-CMP)
2.2 Molecular formula
C9H14N3O8P
2.3 Structural formula
2.4 Relative molecular mass
323.20 (by 2018 international relative atomic mass)
3 Technical requirements
3.1 Sensory requirements
Sensory requirements shall meet the requirements of Table 1.
Appendix A
Inspection method
A.1 General provisions
The reagents and water used in this standard, unless other requirements are specified,
refer to the analytical reagents and the grade-3 water, which is specified in GB/T 6682.
The standard titration solution, standard solution for impurity determination,
preparations and products, which are used in the test, shall be prepared in accordance
with the provisions of GB/T 601, GB/T 602, GB/T 603, unless otherwise specified. The
solution used in the test refers to the aqueous solution, when the solvent is not specified.
A.2 Identification experiment
It is carried out, according to the method specified in GB/T 6040. The infrared spectrum
of the product shall be consistent with the infrared spectrum of the standard product
(see Appendix B, for the infrared spectrum of the standard product).
A.3 Determination of 5'-cytidine monophosphate (C9H14N3O8P) (on a dry basis)
A.3.1 Reagents and materials
A.3.1.1 Water: Grade-1 water in accordance with GB/T 6682-2008.
A.3.1.2 2 mol/L sodium hydroxide solution: Weigh 40 g of solid sodium hydroxide in
a 500 mL volumetric flask. Use water to dissolve it. Dilute it to the mark. Shake well.
A.3.1.3 0.05 mol/L potassium dihydrogen phosphate solution: Weigh 6.8045 g of
potassium dihydrogen phosphate (KH2PO4) into a 1 L volumetric flask. Use water to
dissolve it AND dilute it to the mark. Shake well. Use a 0.45 µm microporous
membrane for filtration. Perform ultrasonic degassing for 30 min, before use.
A.3.1.4 5'-cytidine monophosphate standard substance (purity ≥ 98%).
A.3.1.5 Standard stock solution of 5'-cytidine monophosphate: Accurately weigh 100.0
mg of 5'-cytidine monophosphate standard substance, in a 50 mL volumetric flask. Use
water to dissolve it. Add 1 ~ 2 drops of 2 mol/L sodium hydroxide solution, to help
dissolve. Make the volume to the mark. Mix well. Seal and store it in a 4 °C refrigerator,
to prepare for later use.
A.3.1.6 Standard use solution of 5'-cytidine monophosphate: Accurately pipette 1.0 mL
of 5'-cytidine monophosphate standard stock solution, into a 50 mL volumetric flask.
Use water to dilute it to the mark. Shake well. Use a 0.45 µm microporous membrane
for filtration, before injection.
A.3.2 Instruments and equipment
A.3.2.1 High performance liquid chromatography (ultraviolet detector).
A.3.2.2 Analytical balance (sensitivity 0.0001 g).
A.3.3 Reference liquid chromatography conditions
A.3.3.1 Chromatographic column: C18 (4.6 mm × 250 mm, 5 µm) or equivalent type
column.
A.3.3.2 Mobile phase: 0.05 mol/L KH2PO4 solution + methanol = 95 + 5.
A.3.3.3 Injection volume: 20 µL.
A.3.3.4 Flow rate: 0.8 mL/min.
A.3.3.5 UV detection wavelength: 254 nm.
A.3.3.6 Column oven temperature: 25 °C.
A.3.4 Preparation of specimen
Sample stock solution: Accurately weigh 100.0 mg of the sample into a 50 mL
volumetric flask. Use water to dissolve it. Add 1 ~ 2 drops of 2 mol/L sodium hydroxide
solution, to help dissolve. Make the volume to the mark. Mix well.
Sample use solution: Accurately pipette 1.0 mL of the sample stock solution, into a 50
mL volumetric flask. Use water to make the volume reach to the mark. Shake well. Use
a 0.45 μm microporous membrane for filtration, before injection.
A.3.5 Determination
According to the chromatographic conditions of A.3.3, inject the 5'-cytidine
monophosphate standard solution and the sample use solution, respectively. Based on
the retention time of the standard, determine the chromatographic peak of 5'-cytidine
monophosphate in the sample. According to the peak area of the sample, use the
external standard method, to calculate the content of 5'-cytidine monophosphate in the
sample.
A.3.6 Result calculation
The mass fraction w1 of 5'-cytidine monophosphate content (on a dry basis) is
calculated, according to formula (A.1).
Where:
Delivery: 9 seconds. Download (& Email) true-PDF + Invoice.
Get Quotation: Click GB 1903.33-2022 (Self-service in 1-minute)
Historical versions (Master-website): GB 1903.33-2022
Preview True-PDF (Reload/Scroll-down if blank)
GB 1903.33-2022
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National food safety standard - Food nutritional fortification
substance - 5'-CMP
ISSUED ON: JUNE 30, 2022
IMPLEMENTED ON: DECEMBER 30, 2022
Issued by: National Health Commission of PRC;
State Administration for Market Regulation.
Table of Contents
1 Scope ... 3
2 Chemical name, molecular formula, structural formula, relative molecular mass ... 3
3 Technical requirements ... 3
Appendix A Inspection method ... 5
Appendix B Standard infrared spectrum of 5'-cytidine monophosphate ... 9
National food safety standard - Food nutritional fortification
substance - 5'-CMP
1 Scope
This standard applies to the food nutrition fortifier - 5'-cytidine monophosphate, which
is produced by using yeast ribonucleic acid (RNA) as raw material, through nuclease
enzymatic hydrolysis.
2 Chemical name, molecular formula, structural formula,
relative molecular mass
2.1 Chemical name
5'-cytidine monophosphate (5'-cytidylic acid, 5'-CMP)
2.2 Molecular formula
C9H14N3O8P
2.3 Structural formula
2.4 Relative molecular mass
323.20 (by 2018 international relative atomic mass)
3 Technical requirements
3.1 Sensory requirements
Sensory requirements shall meet the requirements of Table 1.
Appendix A
Inspection method
A.1 General provisions
The reagents and water used in this standard, unless other requirements are specified,
refer to the analytical reagents and the grade-3 water, which is specified in GB/T 6682.
The standard titration solution, standard solution for impurity determination,
preparations and products, which are used in the test, shall be prepared in accordance
with the provisions of GB/T 601, GB/T 602, GB/T 603, unless otherwise specified. The
solution used in the test refers to the aqueous solution, when the solvent is not specified.
A.2 Identification experiment
It is carried out, according to the method specified in GB/T 6040. The infrared spectrum
of the product shall be consistent with the infrared spectrum of the standard product
(see Appendix B, for the infrared spectrum of the standard product).
A.3 Determination of 5'-cytidine monophosphate (C9H14N3O8P) (on a dry basis)
A.3.1 Reagents and materials
A.3.1.1 Water: Grade-1 water in accordance with GB/T 6682-2008.
A.3.1.2 2 mol/L sodium hydroxide solution: Weigh 40 g of solid sodium hydroxide in
a 500 mL volumetric flask. Use water to dissolve it. Dilute it to the mark. Shake well.
A.3.1.3 0.05 mol/L potassium dihydrogen phosphate solution: Weigh 6.8045 g of
potassium dihydrogen phosphate (KH2PO4) into a 1 L volumetric flask. Use water to
dissolve it AND dilute it to the mark. Shake well. Use a 0.45 µm microporous
membrane for filtration. Perform ultrasonic degassing for 30 min, before use.
A.3.1.4 5'-cytidine monophosphate standard substance (purity ≥ 98%).
A.3.1.5 Standard stock solution of 5'-cytidine monophosphate: Accurately weigh 100.0
mg of 5'-cytidine monophosphate standard substance, in a 50 mL volumetric flask. Use
water to dissolve it. Add 1 ~ 2 drops of 2 mol/L sodium hydroxide solution, to help
dissolve. Make the volume to the mark. Mix well. Seal and store it in a 4 °C refrigerator,
to prepare for later use.
A.3.1.6 Standard use solution of 5'-cytidine monophosphate: Accurately pipette 1.0 mL
of 5'-cytidine monophosphate standard stock solution, into a 50 mL volumetric flask.
Use water to dilute it to the mark. Shake well. Use a 0.45 µm microporous membrane
for filtration, before injection.
A.3.2 Instruments and equipment
A.3.2.1 High performance liquid chromatography (ultraviolet detector).
A.3.2.2 Analytical balance (sensitivity 0.0001 g).
A.3.3 Reference liquid chromatography conditions
A.3.3.1 Chromatographic column: C18 (4.6 mm × 250 mm, 5 µm) or equivalent type
column.
A.3.3.2 Mobile phase: 0.05 mol/L KH2PO4 solution + methanol = 95 + 5.
A.3.3.3 Injection volume: 20 µL.
A.3.3.4 Flow rate: 0.8 mL/min.
A.3.3.5 UV detection wavelength: 254 nm.
A.3.3.6 Column oven temperature: 25 °C.
A.3.4 Preparation of specimen
Sample stock solution: Accurately weigh 100.0 mg of the sample into a 50 mL
volumetric flask. Use water to dissolve it. Add 1 ~ 2 drops of 2 mol/L sodium hydroxide
solution, to help dissolve. Make the volume to the mark. Mix well.
Sample use solution: Accurately pipette 1.0 mL of the sample stock solution, into a 50
mL volumetric flask. Use water to make the volume reach to the mark. Shake well. Use
a 0.45 μm microporous membrane for filtration, before injection.
A.3.5 Determination
According to the chromatographic conditions of A.3.3, inject the 5'-cytidine
monophosphate standard solution and the sample use solution, respectively. Based on
the retention time of the standard, determine the chromatographic peak of 5'-cytidine
monophosphate in the sample. According to the peak area of the sample, use the
external standard method, to calculate the content of 5'-cytidine monophosphate in the
sample.
A.3.6 Result calculation
The mass fraction w1 of 5'-cytidine monophosphate content (on a dry basis) is
calculated, according to formula (A.1).
Where:
GB 1903.33-2022
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National food safety standard - Food nutritional fortification
substance - 5'-CMP
ISSUED ON: JUNE 30, 2022
IMPLEMENTED ON: DECEMBER 30, 2022
Issued by: National Health Commission of PRC;
State Administration for Market Regulation.
Table of Contents
1 Scope ... 3
2 Chemical name, molecular formula, structural formula, relative molecular mass ... 3
3 Technical requirements ... 3
Appendix A Inspection method ... 5
Appendix B Standard infrared spectrum of 5'-cytidine monophosphate ... 9
National food safety standard - Food nutritional fortification
substance - 5'-CMP
1 Scope
This standard applies to the food nutrition fortifier - 5'-cytidine monophosphate, which
is produced by using yeast ribonucleic acid (RNA) as raw material, through nuclease
enzymatic hydrolysis.
2 Chemical name, molecular formula, structural formula,
relative molecular mass
2.1 Chemical name
5'-cytidine monophosphate (5'-cytidylic acid, 5'-CMP)
2.2 Molecular formula
C9H14N3O8P
2.3 Structural formula
2.4 Relative molecular mass
323.20 (by 2018 international relative atomic mass)
3 Technical requirements
3.1 Sensory requirements
Sensory requirements shall meet the requirements of Table 1.
Appendix A
Inspection method
A.1 General provisions
The reagents and water used in this standard, unless other requirements are specified,
refer to the analytical reagents and the grade-3 water, which is specified in GB/T 6682.
The standard titration solution, standard solution for impurity determination,
preparations and products, which are used in the test, shall be prepared in accordance
with the provisions of GB/T 601, GB/T 602, GB/T 603, unless otherwise specified. The
solution used in the test refers to the aqueous solution, when the solvent is not specified.
A.2 Identification experiment
It is carried out, according to the method specified in GB/T 6040. The infrared spectrum
of the product shall be consistent with the infrared spectrum of the standard product
(see Appendix B, for the infrared spectrum of the standard product).
A.3 Determination of 5'-cytidine monophosphate (C9H14N3O8P) (on a dry basis)
A.3.1 Reagents and materials
A.3.1.1 Water: Grade-1 water in accordance with GB/T 6682-2008.
A.3.1.2 2 mol/L sodium hydroxide solution: Weigh 40 g of solid sodium hydroxide in
a 500 mL volumetric flask. Use water to dissolve it. Dilute it to the mark. Shake well.
A.3.1.3 0.05 mol/L potassium dihydrogen phosphate solution: Weigh 6.8045 g of
potassium dihydrogen phosphate (KH2PO4) into a 1 L volumetric flask. Use water to
dissolve it AND dilute it to the mark. Shake well. Use a 0.45 µm microporous
membrane for filtration. Perform ultrasonic degassing for 30 min, before use.
A.3.1.4 5'-cytidine monophosphate standard substance (purity ≥ 98%).
A.3.1.5 Standard stock solution of 5'-cytidine monophosphate: Accurately weigh 100.0
mg of 5'-cytidine monophosphate standard substance, in a 50 mL volumetric flask. Use
water to dissolve it. Add 1 ~ 2 drops of 2 mol/L sodium hydroxide solution, to help
dissolve. Make the volume to the mark. Mix well. Seal and store it in a 4 °C refrigerator,
to prepare for later use.
A.3.1.6 Standard use solution of 5'-cytidine monophosphate: Accurately pipette 1.0 mL
of 5'-cytidine monophosphate standard stock solution, into a 50 mL volumetric flask.
Use water to dilute it to the mark. Shake well. Use a 0.45 µm microporous membrane
for filtration, before injection.
A.3.2 Instruments and equipment
A.3.2.1 High performance liquid chromatography (ultraviolet detector).
A.3.2.2 Analytical balance (sensitivity 0.0001 g).
A.3.3 Reference liquid chromatography conditions
A.3.3.1 Chromatographic column: C18 (4.6 mm × 250 mm, 5 µm) or equivalent type
column.
A.3.3.2 Mobile phase: 0.05 mol/L KH2PO4 solution + methanol = 95 + 5.
A.3.3.3 Injection volume: 20 µL.
A.3.3.4 Flow rate: 0.8 mL/min.
A.3.3.5 UV detection wavelength: 254 nm.
A.3.3.6 Column oven temperature: 25 °C.
A.3.4 Preparation of specimen
Sample stock solution: Accurately weigh 100.0 mg of the sample into a 50 mL
volumetric flask. Use water to dissolve it. Add 1 ~ 2 drops of 2 mol/L sodium hydroxide
solution, to help dissolve. Make the volume to the mark. Mix well.
Sample use solution: Accurately pipette 1.0 mL of the sample stock solution, into a 50
mL volumetric flask. Use water to make the volume reach to the mark. Shake well. Use
a 0.45 μm microporous membrane for filtration, before injection.
A.3.5 Determination
According to the chromatographic conditions of A.3.3, inject the 5'-cytidine
monophosphate standard solution and the sample use solution, respectively. Based on
the retention time of the standard, determine the chromatographic peak of 5'-cytidine
monophosphate in the sample. According to the peak area of the sample, use the
external standard method, to calculate the content of 5'-cytidine monophosphate in the
sample.
A.3.6 Result calculation
The mass fraction w1 of 5'-cytidine monophosphate content (on a dry basis) is
calculated, according to formula (A.1).
Where:
Share