1
/
of
7
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
GB 1903.49-2020 English PDF
GB 1903.49-2020 English PDF
Regular price
$115.00 USD
Regular price
Sale price
$115.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click GB 1903.49-2020
Historical versions: GB 1903.49-2020
Preview True-PDF (Reload/Scroll if blank)
GB 1903.49-2020: National food safety standard - Food nutritional fortification substance - Zinc citrate trihydrate
GB 1903.49-2020
GB
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National Food Safety Standard - Food Nutritional
Fortification Substance - Zinc Citrate Trihydrate
食品营养强化剂 柠檬酸锌
ISSUED ON: SEPTEMBER 11, 2020
IMPLEMENTED ON: MARCH 11, 2021
Issued by: National Health Commission of the People’s Republic of China;
State Administration for Market Regulation.
Table of Contents
1 Scope ... 3
2 Chemical Name, Molecular Formula, Structural Formula and Relative
Molecular Mass ... 3
3 Technical Requirements ... 3
Appendix A Inspection Method ... 5
National Food Safety Standard - Food Nutritional
Fortification Substance - Zinc Citrate Trihydrate
1 Scope
This Standard is applicable to food nutritional fortification substance - zinc citrate
trihydrate, which is obtained through chemical synthesis and refining with citric acid
and zinc oxide or zinc carbonate as the main raw materials.
2 Chemical Name, Molecular Formula, Structural
Formula and Relative Molecular Mass
2.1 Chemical Name
Zinc citrate trihydrate
2.2 Molecular Formula
C12H10O14Zn3 nH2O (n = 0, 2 or 3)
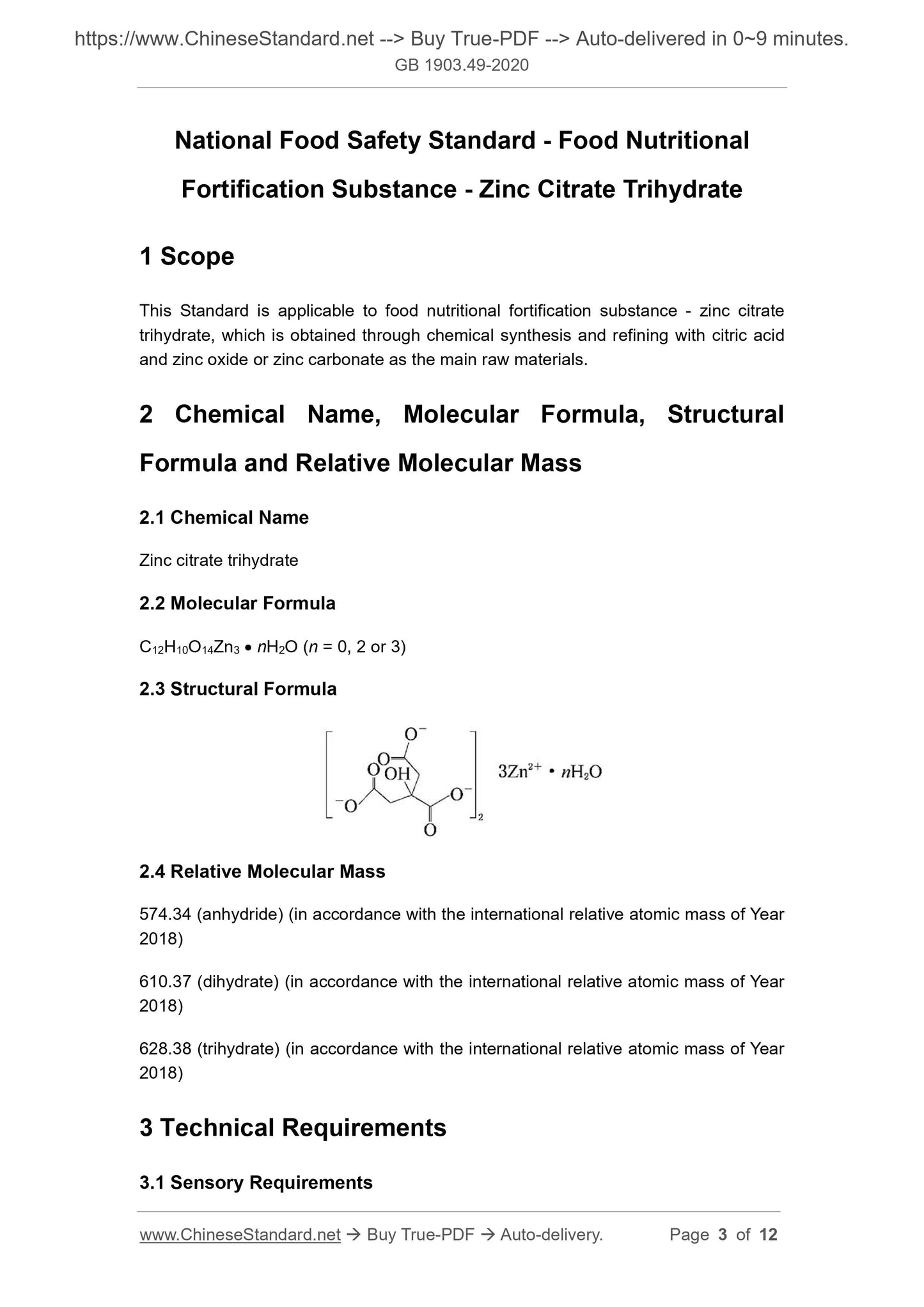
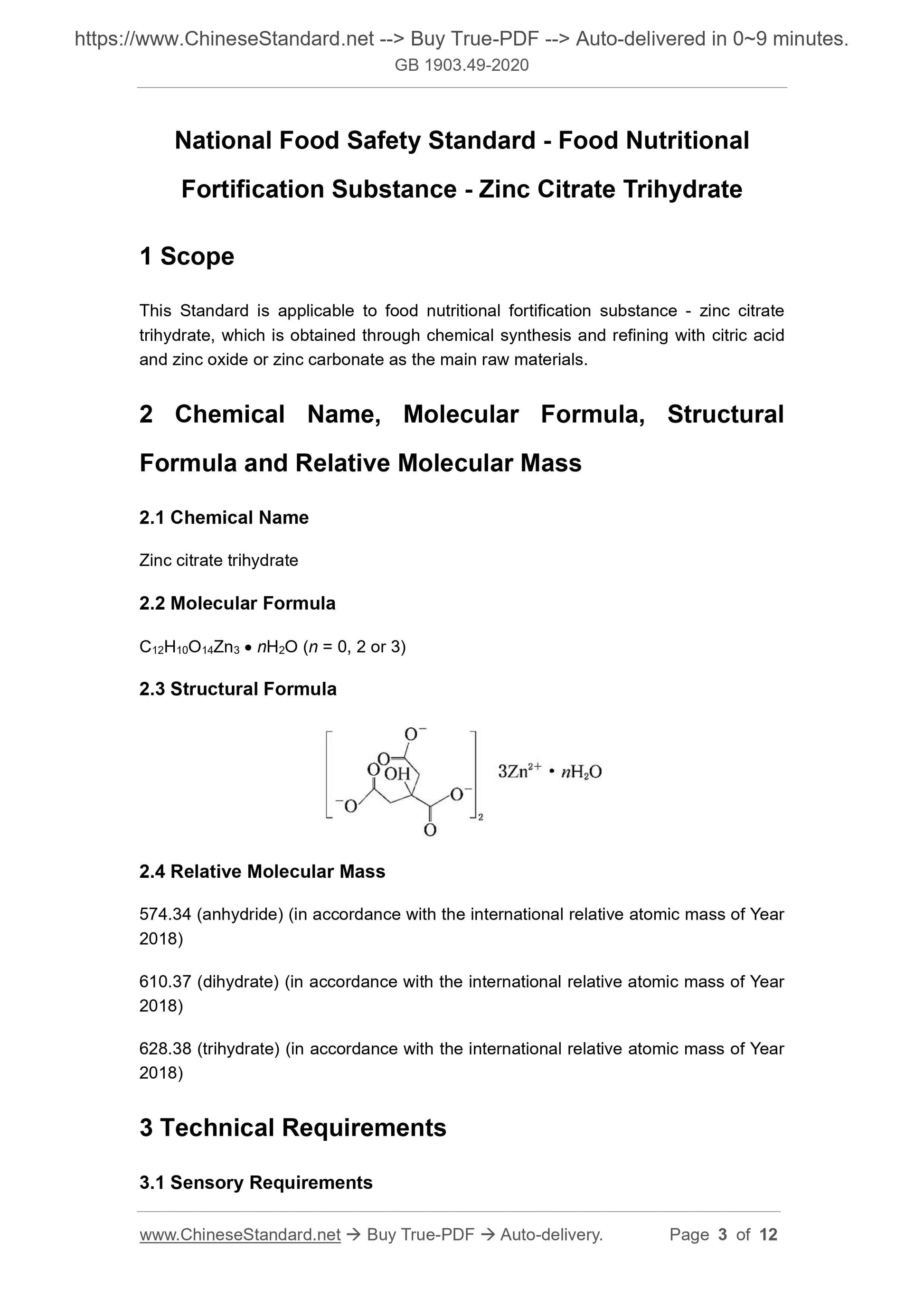
2.3 Structural Formula
2.4 Relative Molecular Mass
574.34 (anhydride) (in accordance with the international relative atomic mass of Year
2018)
610.37 (dihydrate) (in accordance with the international relative atomic mass of Year
2018)
628.38 (trihydrate) (in accordance with the international relative atomic mass of Year
2018)
3 Technical Requirements
3.1 Sensory Requirements
Appendix A
Inspection Method
A.1 General Provisions
When no other requirements are indicated, the reagents and water used in this
Standard refer to analytically pure reagents and Grade-3 water specified in GB/T 6682.
When no other requirements are indicated, the standard titration solutions, and
standard solutions, preparations and products for impurity determination shall be
prepared in accordance with the stipulations of GB/T 601, GB/T 602 and GB/T 603.
When the solvent used for solution preparation is not specified, it refers to aqueous
solution in the tests.
A.2 Identification Test
A.2.1 Reagents and materials
A.2.1.1 Hydrochloric acid solution: 1 + 4.
A.2.1.2 Sulfuric acid solution: 5%.
A.2.1.3 Potassium ferrocyanide solution: 100 g/L. Prepare it right before use.
A.2.1.4 Potassium permanganate solution: 3.2 g/L.
A.2.1.5 Mercury sulfate solution: weigh-take 5 g of mercury oxide; firstly, add 40 mL of
water, then, slowly add 20 mL of concentrated sulfuric acid; stir while adding it, then,
add 40 mL of water; stir to dissolve it.
A.2.1.6 Pyridine-acetic anhydride: 3 + 1.
A.2.2 Identification method
A.2.2.1 Solubility (anhydride and dihydrate)
Slightly soluble in water; soluble in hydrochloric acid solution.
A.2.2.2 Identification of zinc salt
Weigh-take about 0.2 g of the sample, dissolve it in 20 mL of water; add newly prepared
2 mL of potassium ferrocyanide solution, which generates white precipitate. Separate
the precipitate through centrifugation or filtration; add hydrochloric acid solution to the
precipitate, and the precipitate will not dissolve.
A.2.2.3 Identification of citrate
Where,
V1---the volume of the Ethylene diamine tetra-acetic acid (EDTA) standard titration
solution consumed by the titration of the sample solution, expressed in (mL);
V0---the volume of the Ethylene diamine tetra-acetic acid (EDTA) standard titration
solution consumed by the titration of the blank solution, expressed in (mL);
c1---the concentration of the Ethylene diamine tetra-acetic acid (EDTA) standard
titration solution, expressed in (mol/L);
M1---the molar mass of zinc citrate trihydrate, expressed in (g/mol), [M1 (
C12H10O14Zn3 3H2O) = 209.46];
m1---the mass of the sample, expressed in (g);
1,000---conversion factor.
Take the arithmetic mean value of two parallel determination results as the report result.
The absolute difference between two parallel determination results shall be not greater
than 0.3%.
A.4 Determination of Zinc (Zn) Content (calculated as dry basis)
A.4.1 Method summary
Take chrome black T as the indicator solution; use ethylene diamine tetra-acetic acid
(EDTA) standard titration solution to titrate the sample solution. In accordance with the
amount of the ethylene diamine tetra-acetic acid standard titration solution being used,
calculate the content of zinc calculated as Zn.
A.4.2 Reagents and materials
A.4.2.1 Ammonia-ammonium chloride buffer solution (pH 10).
A.4.2.2 Ethylene diamine tetra-acetic acid (EDTA) standard titration solution: c (EDTA)
= 0.05 mol/L.
A.4.2.3 Chrome black T indicator solution.
A.4.3 Analytical procedures
Weigh-take 0.35 g of the sample (accurate to 0.0001 g) dried to a constant weight at
105 °C; add 60 mL of water and 10 mL of ammonia-ammonium chloride buffer solution;
shake to dissolve it. Add a little chrome black T indicator solution; use ethylene diamine
tetra-acetic acid (EDTA) standard titration solution to titrate, until the solution turns from
purple to pure blue. Meanwhile, conduct a blank test.
A.5.4 Result calculation
The solubility of the sample shall be calculated in accordance with Formula (A.3):
Where,
w3---the solubility of the sample, expressed in (g/100 mL);
m3---the mass of the sample, expressed in (g);
m4---the mass of the glass sand core funnel and the precipitate after drying, expressed
in (g);
m5---the mass of the glass sand core funnel, expressed in (g).
Take the arithmetic mean value of parallel determination results as the determination
result. The difference between two parallel determination results shall not exceed 2%
of the arithmetic mean value.
A.6 Determination of Hydrochloric Acid Insoluble Matter
A.6.1 Reagents and materials
A.6.1.1 Hydrochloric acid solution: 6 mol/L.
A.6.1.2 Glass sand core funnel: with a pore size of 4 μm ~ 9 μm.
A.6.2 Instruments and equipment
Electronic balance: with a division value of 1 mg.
A.6.3 Analytical procedures
Weigh-take 5 g of the sample (accurate to 0.001 g); add 10 mL of hydrochloric acid
solution and 50 mL of water. Magnetically heat it up and stir it for 30 min. Let the
obtained solution pass through a glass sand core funnel, which is dried at 105 °C ±
2 °C for 2 h, cooled down and weighed; conduct vacuum filtration. Divide 200 mL of
water into 5 parts to rinse it. The precipitate is dried at 105 °C ± 2 °C for 2 h, cooled
down and weighed.
A.6.4 Result calculation
The mass fraction w4 of hydrochloric acid insoluble matter shall be calculated in
accordance with Formula (A.4):
A.8.1.2 Barium chloride solution: 250 g/L.
A.8.1.3 Potassium sulfate standard solution: after preparing it in accordance with GB/T
602, dilute it to the equivalent of 0.01 mg of sulfate ion per 1 mL.
A.8.2 Analytical procedures
Weigh-take 0.10 g ± 0.01 g of the sample; place it in a 50 mL Nessler colorimetric tube.
Add an appropriate amount of water and 2 mL of hydrochloric acid solution to dissolve
it. Add 5 mL of barium chloride solution; use water to dilute it to 50 mL; shake it well;
place it in a dark place for 10 min. Under a black background, axially observe it;
compare the manifested turbidity with that of the standard turbidity solution.
Standard turbidity solution: measure-take 5 mL of sulfate standard solution and place
it in a 50 mL colorimetric tube. Process it at the same time and in the same mode as
the sample solution.
A.8.3 Result determination ...
Get QUOTATION in 1-minute: Click GB 1903.49-2020
Historical versions: GB 1903.49-2020
Preview True-PDF (Reload/Scroll if blank)
GB 1903.49-2020: National food safety standard - Food nutritional fortification substance - Zinc citrate trihydrate
GB 1903.49-2020
GB
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National Food Safety Standard - Food Nutritional
Fortification Substance - Zinc Citrate Trihydrate
食品营养强化剂 柠檬酸锌
ISSUED ON: SEPTEMBER 11, 2020
IMPLEMENTED ON: MARCH 11, 2021
Issued by: National Health Commission of the People’s Republic of China;
State Administration for Market Regulation.
Table of Contents
1 Scope ... 3
2 Chemical Name, Molecular Formula, Structural Formula and Relative
Molecular Mass ... 3
3 Technical Requirements ... 3
Appendix A Inspection Method ... 5
National Food Safety Standard - Food Nutritional
Fortification Substance - Zinc Citrate Trihydrate
1 Scope
This Standard is applicable to food nutritional fortification substance - zinc citrate
trihydrate, which is obtained through chemical synthesis and refining with citric acid
and zinc oxide or zinc carbonate as the main raw materials.
2 Chemical Name, Molecular Formula, Structural
Formula and Relative Molecular Mass
2.1 Chemical Name
Zinc citrate trihydrate
2.2 Molecular Formula
C12H10O14Zn3 nH2O (n = 0, 2 or 3)
2.3 Structural Formula
2.4 Relative Molecular Mass
574.34 (anhydride) (in accordance with the international relative atomic mass of Year
2018)
610.37 (dihydrate) (in accordance with the international relative atomic mass of Year
2018)
628.38 (trihydrate) (in accordance with the international relative atomic mass of Year
2018)
3 Technical Requirements
3.1 Sensory Requirements
Appendix A
Inspection Method
A.1 General Provisions
When no other requirements are indicated, the reagents and water used in this
Standard refer to analytically pure reagents and Grade-3 water specified in GB/T 6682.
When no other requirements are indicated, the standard titration solutions, and
standard solutions, preparations and products for impurity determination shall be
prepared in accordance with the stipulations of GB/T 601, GB/T 602 and GB/T 603.
When the solvent used for solution preparation is not specified, it refers to aqueous
solution in the tests.
A.2 Identification Test
A.2.1 Reagents and materials
A.2.1.1 Hydrochloric acid solution: 1 + 4.
A.2.1.2 Sulfuric acid solution: 5%.
A.2.1.3 Potassium ferrocyanide solution: 100 g/L. Prepare it right before use.
A.2.1.4 Potassium permanganate solution: 3.2 g/L.
A.2.1.5 Mercury sulfate solution: weigh-take 5 g of mercury oxide; firstly, add 40 mL of
water, then, slowly add 20 mL of concentrated sulfuric acid; stir while adding it, then,
add 40 mL of water; stir to dissolve it.
A.2.1.6 Pyridine-acetic anhydride: 3 + 1.
A.2.2 Identification method
A.2.2.1 Solubility (anhydride and dihydrate)
Slightly soluble in water; soluble in hydrochloric acid solution.
A.2.2.2 Identification of zinc salt
Weigh-take about 0.2 g of the sample, dissolve it in 20 mL of water; add newly prepared
2 mL of potassium ferrocyanide solution, which generates white precipitate. Separate
the precipitate through centrifugation or filtration; add hydrochloric acid solution to the
precipitate, and the precipitate will not dissolve.
A.2.2.3 Identification of citrate
Where,
V1---the volume of the Ethylene diamine tetra-acetic acid (EDTA) standard titration
solution consumed by the titration of the sample solution, expressed in (mL);
V0---the volume of the Ethylene diamine tetra-acetic acid (EDTA) standard titration
solution consumed by the titration of the blank solution, expressed in (mL);
c1---the concentration of the Ethylene diamine tetra-acetic acid (EDTA) standard
titration solution, expressed in (mol/L);
M1---the molar mass of zinc citrate trihydrate, expressed in (g/mol), [M1 (
C12H10O14Zn3 3H2O) = 209.46];
m1---the mass of the sample, expressed in (g);
1,000---conversion factor.
Take the arithmetic mean value of two parallel determination results as the report result.
The absolute difference between two parallel determination results shall be not greater
than 0.3%.
A.4 Determination of Zinc (Zn) Content (calculated as dry basis)
A.4.1 Method summary
Take chrome black T as the indicator solution; use ethylene diamine tetra-acetic acid
(EDTA) standard titration solution to titrate the sample solution. In accordance with the
amount of the ethylene diamine tetra-acetic acid standard titration solution being used,
calculate the content of zinc calculated as Zn.
A.4.2 Reagents and materials
A.4.2.1 Ammonia-ammonium chloride buffer solution (pH 10).
A.4.2.2 Ethylene diamine tetra-acetic acid (EDTA) standard titration solution: c (EDTA)
= 0.05 mol/L.
A.4.2.3 Chrome black T indicator solution.
A.4.3 Analytical procedures
Weigh-take 0.35 g of the sample (accurate to 0.0001 g) dried to a constant weight at
105 °C; add 60 mL of water and 10 mL of ammonia-ammonium chloride buffer solution;
shake to dissolve it. Add a little chrome black T indicator solution; use ethylene diamine
tetra-acetic acid (EDTA) standard titration solution to titrate, until the solution turns from
purple to pure blue. Meanwhile, conduct a blank test.
A.5.4 Result calculation
The solubility of the sample shall be calculated in accordance with Formula (A.3):
Where,
w3---the solubility of the sample, expressed in (g/100 mL);
m3---the mass of the sample, expressed in (g);
m4---the mass of the glass sand core funnel and the precipitate after drying, expressed
in (g);
m5---the mass of the glass sand core funnel, expressed in (g).
Take the arithmetic mean value of parallel determination results as the determination
result. The difference between two parallel determination results shall not exceed 2%
of the arithmetic mean value.
A.6 Determination of Hydrochloric Acid Insoluble Matter
A.6.1 Reagents and materials
A.6.1.1 Hydrochloric acid solution: 6 mol/L.
A.6.1.2 Glass sand core funnel: with a pore size of 4 μm ~ 9 μm.
A.6.2 Instruments and equipment
Electronic balance: with a division value of 1 mg.
A.6.3 Analytical procedures
Weigh-take 5 g of the sample (accurate to 0.001 g); add 10 mL of hydrochloric acid
solution and 50 mL of water. Magnetically heat it up and stir it for 30 min. Let the
obtained solution pass through a glass sand core funnel, which is dried at 105 °C ±
2 °C for 2 h, cooled down and weighed; conduct vacuum filtration. Divide 200 mL of
water into 5 parts to rinse it. The precipitate is dried at 105 °C ± 2 °C for 2 h, cooled
down and weighed.
A.6.4 Result calculation
The mass fraction w4 of hydrochloric acid insoluble matter shall be calculated in
accordance with Formula (A.4):
A.8.1.2 Barium chloride solution: 250 g/L.
A.8.1.3 Potassium sulfate standard solution: after preparing it in accordance with GB/T
602, dilute it to the equivalent of 0.01 mg of sulfate ion per 1 mL.
A.8.2 Analytical procedures
Weigh-take 0.10 g ± 0.01 g of the sample; place it in a 50 mL Nessler colorimetric tube.
Add an appropriate amount of water and 2 mL of hydrochloric acid solution to dissolve
it. Add 5 mL of barium chloride solution; use water to dilute it to 50 mL; shake it well;
place it in a dark place for 10 min. Under a black background, axially observe it;
compare the manifested turbidity with that of the standard turbidity solution.
Standard turbidity solution: measure-take 5 mL of sulfate standard solution and place
it in a 50 mL colorimetric tube. Process it at the same time and in the same mode as
the sample solution.
A.8.3 Result determination ...
Share