1
/
of
8
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
GB 29922-2013 English PDF
GB 29922-2013 English PDF
Regular price
$85.00 USD
Regular price
Sale price
$85.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click GB 29922-2013
Historical versions: GB 29922-2013
Preview True-PDF (Reload/Scroll if blank)
GB 29922-2013: National Food Safety Standard -- General Rules on Formula Food for Medical Use
GB 29922-2013
GB
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National food safety standard -
General principles for formula foods of special
medical purpose
ISSUED ON. DECEMBER 26, 2013
IMPLEMENTED ON. JULY 01, 2014
Issued by. National Health and Family Planning
Commission of the People’s Republic of China
Table of Contents
1 Scope ... 3
2 Terms and definitions ... 3
3 Technical requirements ... 3
4 Others ... 11
Annex A Common specific whole nutrient formula foods ... 13
Annex B Applicable amino acid for formula foods of special medical purpose
... 14
National food safety standard -
General principles for formula foods of special
medical purpose
1 Scope
This Standard is applicable to formula foods of special medical purpose for
people older than 1 year old.
2 Terms and definitions
2.1 Formula foods of special medical purpose
Formula foods specially processed and prepared in order to meet special needs
for nutrient or diet of those suffering from food intake restriction, disorder of
digestive absorption, disorder of metabolic or certain disease. Such foods must
be used alone or together with other foods under the guidance of doctor or
clinical nutritionist.
2.1.1 Whole nutrient formula foods
Formula foods of special medical purpose that may be used as single nutrition
source to meet the nutritional requirements of the target group.
2.1.2 Specific whole nutrient formula foods
Formula foods of special medical purpose that may be used as single nutrition
source to meet the nutritional requirements of the target group of certain
disease or medical state.
2.1.3 Non-whole nutrient formula foods
Formula foods of special medical purpose that may meet the partial nutritional
requirements of the target group and is not applicable to be regarded as single
nutrition source.
3 Technical requirements
3.1 Basic requirements
The formula of formula foods of special medical purpose shall be based on the
research results of medicine and/or nutrition, and their safety and clinical
application (effectiveness) shall be scientifically verified.
The production condition of formula foods of special medical purpose shall
meet the relevant national regulations.
3.2 Material requirements
The material adopted in formula foods of special medical purpose shall meet
the corresponding standards and/or the relevant requirements, and any
substance endangering the health of eaters is forbidden.
3.3 Sensory requirements
The color and luster, taste, smell, texture state and reconstituability of formula
foods of special medical purpose shall meet the characteristic of corresponding
product which is free from the extrinsic impurity in normal sight.
3.4 Nutrient
3.4.1 Whole nutrient formula foods which are applicable to the group of 1 ~ 10
years old.
3.4.1.1 As for whole nutrient formula foods which are applicable to the group of
1 ~ 10 years old, the energy contained shall not be lower than 250 kJ (60 kcal)
per 100 mL (liquid or dissolvable product under the instant state) or per 100 g
(non-liquid product that can be eat directly). The energy shall be calculated by
multiplying the contents of protein, fat and carbohydrates in 100 mL of product
with the respective corresponding energy coefficients of 17 kJ/g, 37 kJ/g and
17 kJ/g (the energy coefficient of dietary fiber, which is calculated according to
50 % of the energy coefficient of carbohydrates) to obtain a sum expressed in
kJ/100 mL or kJ/100 g, and this sum shall be divided by 4.184 to obtain a value
expressed in kcal/100 mL or kcal/100 g.
3.4.1.2 As for whole nutrient formula foods which are applicable to the group of
1 ~ 10 years old, the content of protein shall not be lower than 0.5 g/100 kJ (2
g/100 kcal), where the proportion of high-quality protein shall not be lower than
50 %. The protein shall be inspected according to GB 5009.5.
3.4.1.3 As for whole nutrient formula foods which are applicable to the group of
1 ~ 10 years old, the energy supply ratio of linoleic acid shall not be lower than
2.5 % and the energy supply ratio of α-linoleic acid shall not be lower than 0.4 %.
The fatty acid shall be inspected according to GB 5413.27.
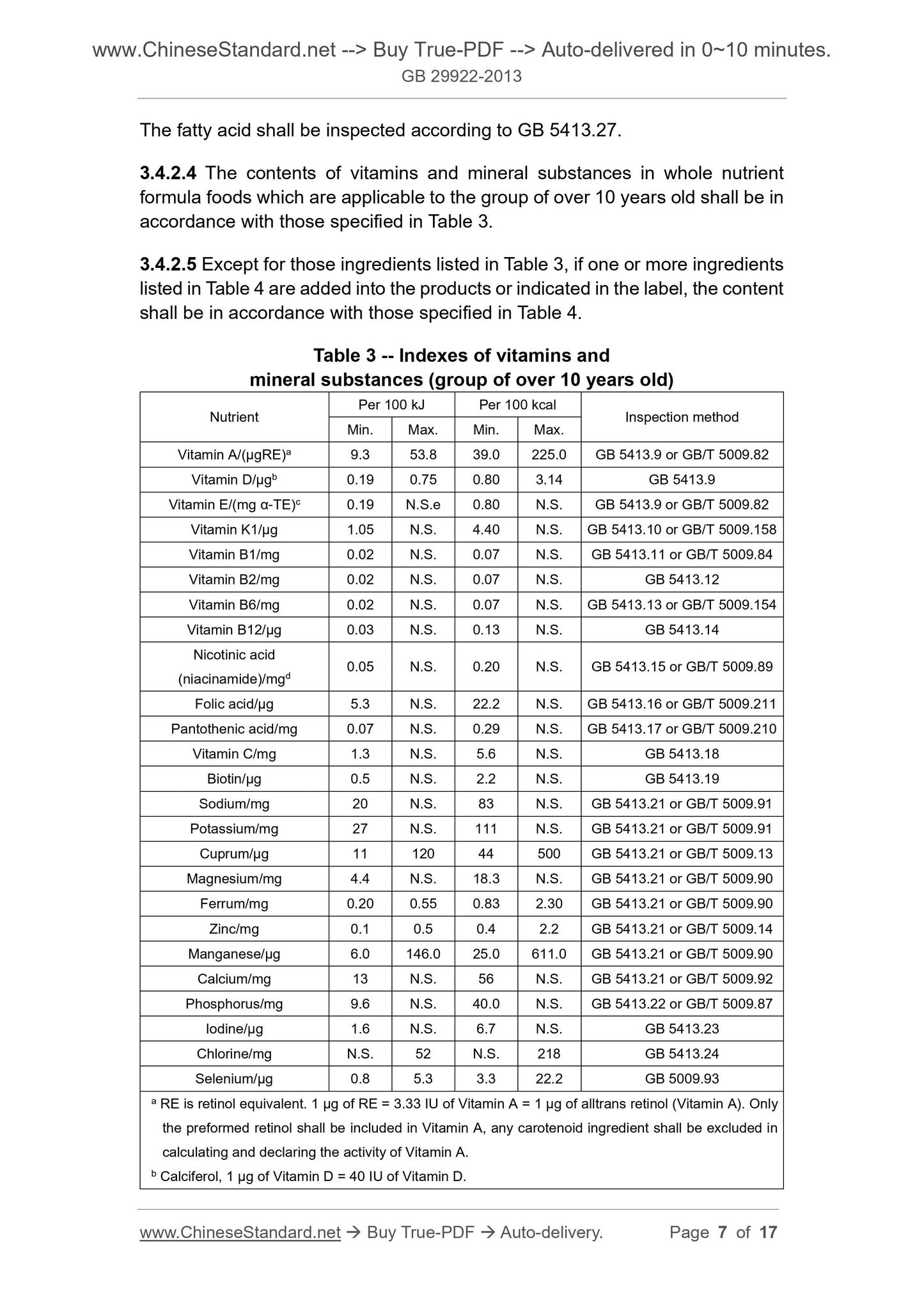
3.4.1.4 The contents of vitamins and mineral substances in whole nutrient
formula foods that are applicable to the group of 1 ~ 10 years old shall be in
The fatty acid shall be inspected according to GB 5413.27.
3.4.2.4 The contents of vitamins and mineral substances in whole nutrient
formula foods which are applicable to the group of over 10 years old shall be in
accordance with those specified in Table 3.
3.4.2.5 Except for those ingredients listed in Table 3, if one or more ingredients
listed in Table 4 are added into the products or indicated in the label, the content
shall be in accordance with those specified in Table 4.
Table 3 -- Indexes of vitamins and
mineral substances (group of over 10 years old)
Nutrient Per 100 kJ Per 100 kcal Inspection method Min. Max. Min. Max.
Vitamin A/(μgRE)a 9.3 53.8 39.0 225.0 GB 5413.9 or GB/T 5009.82
Vitamin D/μgb 0.19 0.75 0.80 3.14 GB 5413.9
Vitamin E/(mg α-TE)c 0.19 N.S.e 0.80 N.S. GB 5413.9 or GB/T 5009.82
Vitamin K1/μg 1.05 N.S. 4.40 N.S. GB 5413.10 or GB/T 5009.158
Vitamin B1/mg 0.02 N.S. 0.07 N.S. GB 5413.11 or GB/T 5009.84
Vitamin B2/mg 0.02 N.S. 0.07 N.S. GB 5413.12
Vitamin B6/mg 0.02 N.S. 0.07 N.S. GB 5413.13 or GB/T 5009.154
Vitamin B12/μg 0.03 N.S. 0.13 N.S. GB 5413.14
Nicotinic acid
(niacinamide)/mgd 0.05 N.S. 0.20 N.S. GB 5413.15 or GB/T 5009.89
Folic acid/μg 5.3 N.S. 22.2 N.S. GB 5413.16 or GB/T 5009.211
Pantothenic acid/mg 0.07 N.S. 0.29 N.S. GB 5413.17 or GB/T 5009.210
Vitamin C/mg 1.3 N.S. 5.6 N.S. GB 5413.18
Biotin/μg 0.5 N.S. 2.2 N.S. GB 5413.19
Sodium/mg 20 N.S. 83 N.S. GB 5413.21 or GB/T 5009.91
Potassium/mg 27 N.S. 111 N.S. GB 5413.21 or GB/T 5009.91
Cuprum/µg 11 120 44 500 GB 5413.21 or GB/T 5009.13
Magnesium/mg 4.4 N.S. 18.3 N.S. GB 5413.21 or GB/T 5009.90
Ferrum/mg 0.20 0.55 0.83 2.30 GB 5413.21 or GB/T 5009.90
Zinc/mg 0.1 0.5 0.4 2.2 GB 5413.21 or GB/T 5009.14
Manganese/µg 6.0 146.0 25.0 611.0 GB 5413.21 or GB/T 5009.90
Calcium/mg 13 N.S. 56 N.S. GB 5413.21 or GB/T 5009.92
Phosphorus/mg 9.6 N.S. 40.0 N.S. GB 5413.22 or GB/T 5009.87
Iodine/µg 1.6 N.S. 6.7 N.S. GB 5413.23
Chlorine/mg N.S. 52 N.S. 218 GB 5413.24
Selenium/µg 0.8 5.3 3.3 22.2 GB 5009.93
a RE is retinol equivalent. 1 μg of RE = 3.33 IU of Vitamin A = 1 μg of alltrans retinol (Vitamin A). Only
the preformed retinol shall be included in Vitamin A, any carotenoid ingredient shall be excluded in
calculating and declaring the activity of Vitamin A.
b Calciferol, 1 μg of Vitamin D = 40 IU of Vitamin D.
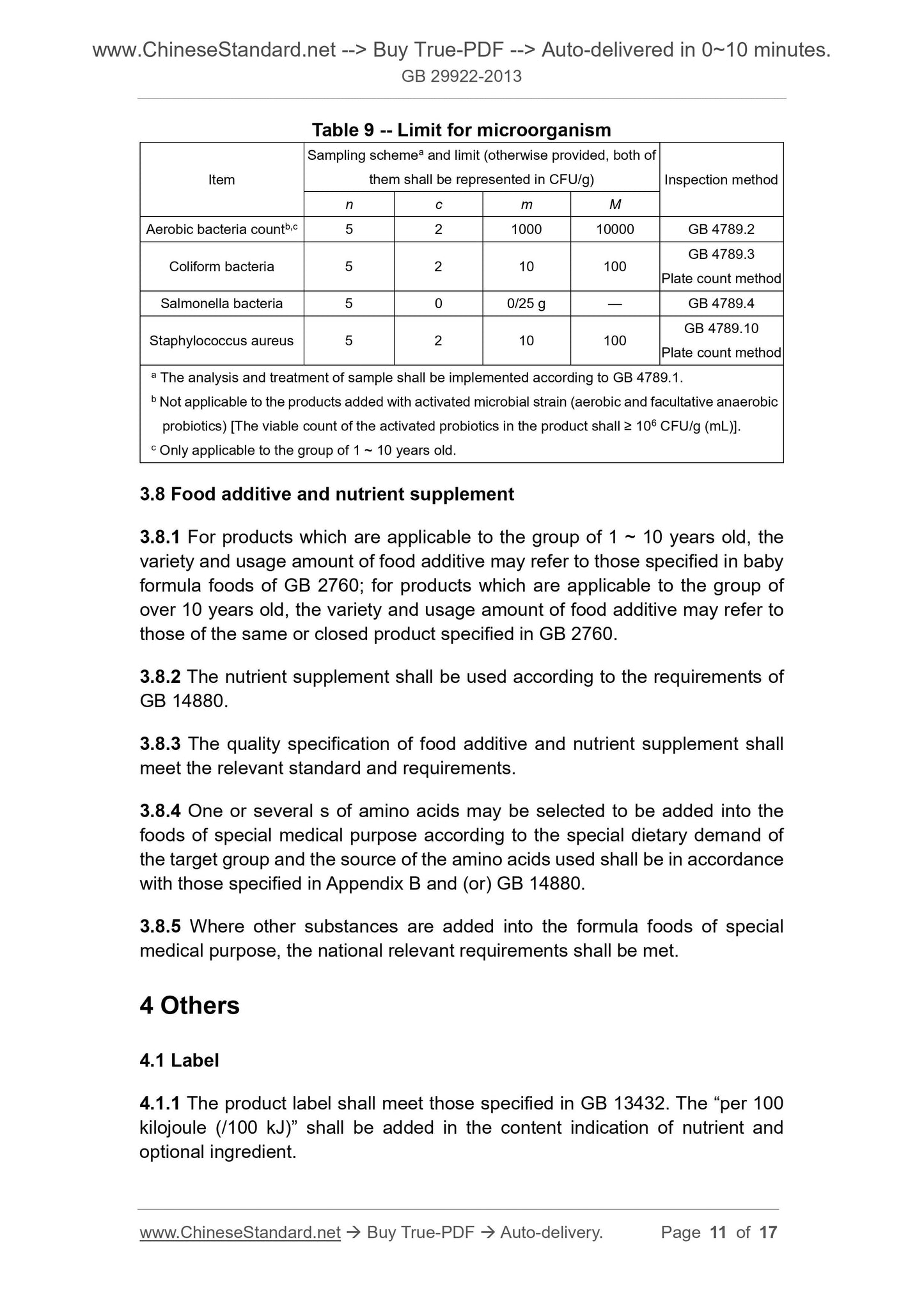
Table 9 -- Limit for microorganism
Item
Sampling schemea and limit (otherwise provided, both of
them shall be represented in CFU/g) Inspection method
n c m M
Aerobic bacteria countb,c 5 2 1000 10000 GB 4789.2
Coliform bacteria 5 2 10 100 GB 4789.3 Plate count method
Salmonella bacteria 5 0 0/25 g — GB 4789.4
Staphylococcus aureus 5 2 10 100 GB 4789.10 Plate count method
a The analysis and treatment of sample shall be implemented according to GB 4789.1.
b Not applicable to the products added with activated microbial strain (aerobic and facultative anaerobic
probiotics) [The viable count of the activated probiotics in the product shall ≥ 106 CFU/g (mL)].
c Only applicable to the group of 1 ~ 10 years old.
3.8 Food...
Get QUOTATION in 1-minute: Click GB 29922-2013
Historical versions: GB 29922-2013
Preview True-PDF (Reload/Scroll if blank)
GB 29922-2013: National Food Safety Standard -- General Rules on Formula Food for Medical Use
GB 29922-2013
GB
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National food safety standard -
General principles for formula foods of special
medical purpose
ISSUED ON. DECEMBER 26, 2013
IMPLEMENTED ON. JULY 01, 2014
Issued by. National Health and Family Planning
Commission of the People’s Republic of China
Table of Contents
1 Scope ... 3
2 Terms and definitions ... 3
3 Technical requirements ... 3
4 Others ... 11
Annex A Common specific whole nutrient formula foods ... 13
Annex B Applicable amino acid for formula foods of special medical purpose
... 14
National food safety standard -
General principles for formula foods of special
medical purpose
1 Scope
This Standard is applicable to formula foods of special medical purpose for
people older than 1 year old.
2 Terms and definitions
2.1 Formula foods of special medical purpose
Formula foods specially processed and prepared in order to meet special needs
for nutrient or diet of those suffering from food intake restriction, disorder of
digestive absorption, disorder of metabolic or certain disease. Such foods must
be used alone or together with other foods under the guidance of doctor or
clinical nutritionist.
2.1.1 Whole nutrient formula foods
Formula foods of special medical purpose that may be used as single nutrition
source to meet the nutritional requirements of the target group.
2.1.2 Specific whole nutrient formula foods
Formula foods of special medical purpose that may be used as single nutrition
source to meet the nutritional requirements of the target group of certain
disease or medical state.
2.1.3 Non-whole nutrient formula foods
Formula foods of special medical purpose that may meet the partial nutritional
requirements of the target group and is not applicable to be regarded as single
nutrition source.
3 Technical requirements
3.1 Basic requirements
The formula of formula foods of special medical purpose shall be based on the
research results of medicine and/or nutrition, and their safety and clinical
application (effectiveness) shall be scientifically verified.
The production condition of formula foods of special medical purpose shall
meet the relevant national regulations.
3.2 Material requirements
The material adopted in formula foods of special medical purpose shall meet
the corresponding standards and/or the relevant requirements, and any
substance endangering the health of eaters is forbidden.
3.3 Sensory requirements
The color and luster, taste, smell, texture state and reconstituability of formula
foods of special medical purpose shall meet the characteristic of corresponding
product which is free from the extrinsic impurity in normal sight.
3.4 Nutrient
3.4.1 Whole nutrient formula foods which are applicable to the group of 1 ~ 10
years old.
3.4.1.1 As for whole nutrient formula foods which are applicable to the group of
1 ~ 10 years old, the energy contained shall not be lower than 250 kJ (60 kcal)
per 100 mL (liquid or dissolvable product under the instant state) or per 100 g
(non-liquid product that can be eat directly). The energy shall be calculated by
multiplying the contents of protein, fat and carbohydrates in 100 mL of product
with the respective corresponding energy coefficients of 17 kJ/g, 37 kJ/g and
17 kJ/g (the energy coefficient of dietary fiber, which is calculated according to
50 % of the energy coefficient of carbohydrates) to obtain a sum expressed in
kJ/100 mL or kJ/100 g, and this sum shall be divided by 4.184 to obtain a value
expressed in kcal/100 mL or kcal/100 g.
3.4.1.2 As for whole nutrient formula foods which are applicable to the group of
1 ~ 10 years old, the content of protein shall not be lower than 0.5 g/100 kJ (2
g/100 kcal), where the proportion of high-quality protein shall not be lower than
50 %. The protein shall be inspected according to GB 5009.5.
3.4.1.3 As for whole nutrient formula foods which are applicable to the group of
1 ~ 10 years old, the energy supply ratio of linoleic acid shall not be lower than
2.5 % and the energy supply ratio of α-linoleic acid shall not be lower than 0.4 %.
The fatty acid shall be inspected according to GB 5413.27.
3.4.1.4 The contents of vitamins and mineral substances in whole nutrient
formula foods that are applicable to the group of 1 ~ 10 years old shall be in
The fatty acid shall be inspected according to GB 5413.27.
3.4.2.4 The contents of vitamins and mineral substances in whole nutrient
formula foods which are applicable to the group of over 10 years old shall be in
accordance with those specified in Table 3.
3.4.2.5 Except for those ingredients listed in Table 3, if one or more ingredients
listed in Table 4 are added into the products or indicated in the label, the content
shall be in accordance with those specified in Table 4.
Table 3 -- Indexes of vitamins and
mineral substances (group of over 10 years old)
Nutrient Per 100 kJ Per 100 kcal Inspection method Min. Max. Min. Max.
Vitamin A/(μgRE)a 9.3 53.8 39.0 225.0 GB 5413.9 or GB/T 5009.82
Vitamin D/μgb 0.19 0.75 0.80 3.14 GB 5413.9
Vitamin E/(mg α-TE)c 0.19 N.S.e 0.80 N.S. GB 5413.9 or GB/T 5009.82
Vitamin K1/μg 1.05 N.S. 4.40 N.S. GB 5413.10 or GB/T 5009.158
Vitamin B1/mg 0.02 N.S. 0.07 N.S. GB 5413.11 or GB/T 5009.84
Vitamin B2/mg 0.02 N.S. 0.07 N.S. GB 5413.12
Vitamin B6/mg 0.02 N.S. 0.07 N.S. GB 5413.13 or GB/T 5009.154
Vitamin B12/μg 0.03 N.S. 0.13 N.S. GB 5413.14
Nicotinic acid
(niacinamide)/mgd 0.05 N.S. 0.20 N.S. GB 5413.15 or GB/T 5009.89
Folic acid/μg 5.3 N.S. 22.2 N.S. GB 5413.16 or GB/T 5009.211
Pantothenic acid/mg 0.07 N.S. 0.29 N.S. GB 5413.17 or GB/T 5009.210
Vitamin C/mg 1.3 N.S. 5.6 N.S. GB 5413.18
Biotin/μg 0.5 N.S. 2.2 N.S. GB 5413.19
Sodium/mg 20 N.S. 83 N.S. GB 5413.21 or GB/T 5009.91
Potassium/mg 27 N.S. 111 N.S. GB 5413.21 or GB/T 5009.91
Cuprum/µg 11 120 44 500 GB 5413.21 or GB/T 5009.13
Magnesium/mg 4.4 N.S. 18.3 N.S. GB 5413.21 or GB/T 5009.90
Ferrum/mg 0.20 0.55 0.83 2.30 GB 5413.21 or GB/T 5009.90
Zinc/mg 0.1 0.5 0.4 2.2 GB 5413.21 or GB/T 5009.14
Manganese/µg 6.0 146.0 25.0 611.0 GB 5413.21 or GB/T 5009.90
Calcium/mg 13 N.S. 56 N.S. GB 5413.21 or GB/T 5009.92
Phosphorus/mg 9.6 N.S. 40.0 N.S. GB 5413.22 or GB/T 5009.87
Iodine/µg 1.6 N.S. 6.7 N.S. GB 5413.23
Chlorine/mg N.S. 52 N.S. 218 GB 5413.24
Selenium/µg 0.8 5.3 3.3 22.2 GB 5009.93
a RE is retinol equivalent. 1 μg of RE = 3.33 IU of Vitamin A = 1 μg of alltrans retinol (Vitamin A). Only
the preformed retinol shall be included in Vitamin A, any carotenoid ingredient shall be excluded in
calculating and declaring the activity of Vitamin A.
b Calciferol, 1 μg of Vitamin D = 40 IU of Vitamin D.
Table 9 -- Limit for microorganism
Item
Sampling schemea and limit (otherwise provided, both of
them shall be represented in CFU/g) Inspection method
n c m M
Aerobic bacteria countb,c 5 2 1000 10000 GB 4789.2
Coliform bacteria 5 2 10 100 GB 4789.3 Plate count method
Salmonella bacteria 5 0 0/25 g — GB 4789.4
Staphylococcus aureus 5 2 10 100 GB 4789.10 Plate count method
a The analysis and treatment of sample shall be implemented according to GB 4789.1.
b Not applicable to the products added with activated microbial strain (aerobic and facultative anaerobic
probiotics) [The viable count of the activated probiotics in the product shall ≥ 106 CFU/g (mL)].
c Only applicable to the group of 1 ~ 10 years old.
3.8 Food...
Share