1
/
of
7
www.ChineseStandard.us -- Field Test Asia Pte. Ltd.
GB/T 6820-2016 English PDF (GB/T6820-2016)
GB/T 6820-2016 English PDF (GB/T6820-2016)
Regular price
$165.00
Regular price
Sale price
$165.00
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
GB/T 6820-2016: Ethanol for industrial use
Delivery: 9 seconds. Download (& Email) true-PDF + Invoice.
Get Quotation: Click GB/T 6820-2016 (Self-service in 1-minute)
Historical versions (Master-website): GB/T 6820-2016
Preview True-PDF (Reload/Scroll-down if blank)
GB/T 6820-2016
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
ICS 71.080.60
G 17
Replacing GB/T 6820-1992
Ethanol for industrial use
ISSUED ON: DECEMBER 13, 2016
IMPLEMENTED ON: JULY 01, 2017
Issued by: General Administration of Quality Supervision, Inspection and
Quarantine of PRC;
Standardization Administration of PRC.
Table of Contents
Foreword ... 3
1 Scope ... 5
2 Normative references ... 5
3 Product categories ... 6
4 Requirements ... 6
5 Test methods ... 7
6 Inspection rules ... 13
7 Marking, packaging, storage and transportation ... 14
8 Safety ... 15
Appendix A (Normative) Typical gas chromatograms and retention times for the
determination of methanol, isopropanol, n-propanol, acetate, C4 + C5 alcohols
... 17
Ethanol for industrial use
1 Scope
This standard specifies the product classification, requirements, test methods,
inspection rules and signs, packaging, storage, transportation and safety of
ethanol for industrial use.
This standard applies to ethanol for industrial use.
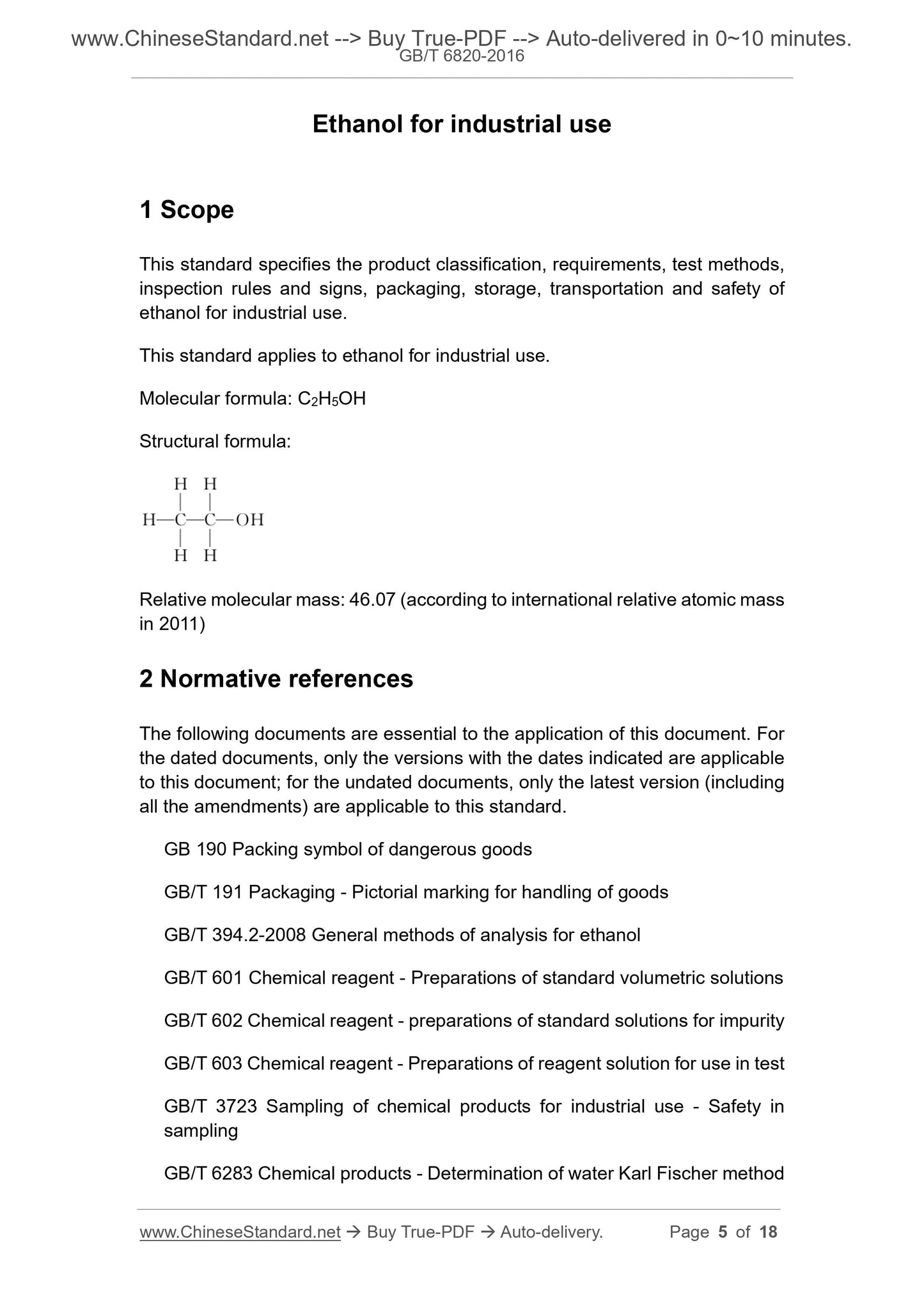
Molecular formula: C2H5OH
Structural formula:
Relative molecular mass: 46.07 (according to international relative atomic mass
in 2011)
2 Normative references
The following documents are essential to the application of this document. For
the dated documents, only the versions with the dates indicated are applicable
to this document; for the undated documents, only the latest version (including
all the amendments) are applicable to this standard.
GB 190 Packing symbol of dangerous goods
GB/T 191 Packaging - Pictorial marking for handling of goods
GB/T 394.2-2008 General methods of analysis for ethanol
GB/T 601 Chemical reagent - Preparations of standard volumetric solutions
GB/T 602 Chemical reagent - preparations of standard solutions for impurity
GB/T 603 Chemical reagent - Preparations of reagent solution for use in test
GB/T 3723 Sampling of chemical products for industrial use - Safety in
sampling
GB/T 6283 Chemical products - Determination of water Karl Fischer method
Determine it according to the method specified in Chapter 11 of GB/T 394.2-
2008.
5.7 Determination of aldehyde content
Determine it according to the method specified in Chapter 8 of GB/T 394.2-
2008. Use the iodine method as the arbitration method.
5.8 Determination of methanol, isopropanol, n-propanol, acetate, C4 + C5
alcohol content
5.8.1 Summary of method
Use gas chromatography, under the selected chromatographic conditions, to
separate the specimen by a chromatographic column. Use a hydrogen flame
ionization detector to detect it. Use the internal standard method, external
standard method, or corrected area normalization method to quantify it. Among
them, use the internal standard method as the arbitration method.
5.8.2 Reagents and solutions
5.8.2.1 Reference ethanol: 95% ethanol by volume, the limit of major impurities
is specified as follows: methanol is less than 2 mg/L; isopropanol is less than 2
mg/L; n-propanol is less than 2 mg/L; higher alcohol (C4 + C5 alcohol ) is less
than 1 mg/L; ester is less than 1 mg/L. When using this method for detection, it
shall not have impurity which may interfere with the internal standard.
5.8.2.2 Standard solution: Weigh 1 g of each of chromatographically pure
methanol, isopropanol, n-propanol, ethyl acetate, isopropyl acetate, 2-butanol,
iso-butanol, n-butanol, isoamyl alcohol, n-pentanol (accurate to 0.0001 g) in a
1000 mL volumetric flask. Use reference ethanol to make its volume reach to
the mark. After shaking it uniformly, it obtains the 1 g/L standard solution.
5.8.2.3 Internal standard solution: Weigh 1 g (accurate to 0.0001 g) of
chromatographic pure n-hexane in a 1000 mL volumetric flask. Use reference
ethanol to make its volume reach to the mark. After shaking it uniformly, it
obtains the 1 g/L internal standard solution.
5.8.2.4 Nitrogen: The volume fraction is not less than 99.95%; it is dried and
purified by silica gel and molecular sieve.
5.8.2.5 Hydrogen: The volume fraction is not less than 99.9%; it is dried and
purified by silica gel and molecular sieve.
5.8.2.6 Air: It is dried and purified by silica gel and molecular sieve.
5.8.3 Instruments and equipment
Take a small amount of the ethanol specimen to be tested in a 10 mL volumetric
flask. Accurately add 0.50 mL of the internal standard solution. Then use the
specimen to be tested to dilute it to the mark. Mix it uniformly. Inject 1 μL of
sample.
Use the retention time of the component peak and the internal standard peak
for qualitative purpose. Use the ratio of the peak area to calculate the content
of each component.
5.8.5.2 External standard method
5.8.5.2.1 Determination of correction factors
Accurately pipette 0.50 mL of the standard solution into a 10 mL volumetric flask.
Use the standard ethanol to dilute it to the mark. Mix it uniformly. Inject 1 μL of
sample. Use the peak area to calculate the correction factor f of each
component.
5.8.5.2.2 Determination of specimen
Under the same instrument conditions as the determination of the correction
factor, take a small amount of the ethanol specimen to be tested and accurately
inject 1 μL of sample.
Use the retention time of the component peak for qualitative purpose. Use the
peak area to calculate the content of each component.
5.8.5.3 Corrected area normalization method
5.8.5.3.1 Determination of correction factors
Accurately pipette 0.50 mL of standard solution into a 10 mL volumetric flask.
Use the standard ethanol to dilute it to the mark. Mix it uniformly. Accurately
inject 1 μL of sample. Use the peak area to calculate the correction factor f for
the relative ethanol of each component except ethanol.
5.8.5.3.2 Determination of specimen
Take a small amount of ethanol specimen to be tested and accurately inject 1
μL of specimen.
Use the retention time of the component peak for qualitative purpose. Use the
ratio of the peak area to calculate the content of each component.
5.8.6 Calculation of results
5.8.6.1 Internal standard method (arbitration method)
5.8.6.1.1 The relative correction factor fi for component i is calculated according
reporting result. The absolute value of the difference between the two parallel
determinations of all components shall meet the requirements of Table 3.
Table 3 -- Maximum allowable differences between two parallel
determinations of each component in the determination
Content of component More than or equal to 10 mg/L
Between 10 mg/L and 5
mg/L
Less than or equal to
5 mg/L
Maximum allowable difference X x 10% X x 20% X x 50%
5.9 Determination of oxidation time of potassium permanganate
Determine it according to the method as specified in Chapter 7 of GB/T 394.2-
2008.
5.10 Determination of evaporation residue
Determine it according to GB/T 6324.2.
Take the arithmetic mean of the two parallel determination results as the
determination result. The difference between the two parallel determination
results is not greater than 2 mg/L.
5.11 Determination of chroma in sulfuric acid test
Determine it according to the method specified in Chapter 6 of GB/T 394.2-
2008.
5.12 Water miscibility test
Determine it according to the method specified in GB/T 6324.1. Choose the
ratio of specimen and water miscibility at 1 + 9 and 1 + 19, respectively.
6 Inspection rules
6.1 Inspection is divided into exit-factory inspection and type inspection. The
appearance and the chromaticity, ethanol, acid, aldehyde, methanol,
isopropanol, n-propanol, acetate, C4 + C5 alcohol, oxidation time of potassium
permanganate and moisture in Table 1 are the exit-factory inspection items and
shall be inspected batch by batch. In the case of normal production, type
inspection shall be carried out at least once a month. Type inspection shall also
be carried out in one of the following cases:
a) In case of update of key production processes;
b) In case of change of the main raw materials;
c) In case of production restoration after suspension;
of the manufacturer. The manufacturer shall ensure that each batch of exit-
factory products meets the requirements of this standard and is accompanied
by a quality certificate in a certain format, which includes:
a) Product name;
b) Product model and grade;
c) Name and address of manufacturer;
d) Production date or batch number;
e) No. of this standard;
f) Industrial product production license marking and number.
7.2 Packaging
Ethanol for industrial use shall be stored in a clean, dry steel tanker or other
container that can guarantee quality.
7.3 Storage
Ethanol for industrial use shall be stored in a cool, dry, ventilated environment
which is isolated from heat sources and fires.
7.4 Transport
Ethanol for industrial use is a flammable and dangerous product. It must not be
exposed to sunlight during transportation; it shall not be stacked with flammable
materials and oxides. It shall strictly follow the relevant provisions for the
transportation of hazardous chemicals.
8 Safety
8.1 Warning of danger
Ethanol for industrial use is an irritating, flammable liquid. The melting point is -
114.3 °C; the boiling point is 78.4 °C; the flash point is 12 °C; the ignition
temperature is 363 °C. Its vapor and air may form explosive mixture; the
explosion limit is 3.3% (volume fraction) ~ 19.0% (volume fraction). When
exposed to open flame and high heat, it may cause combustion and explosion.
Contact with an oxidant may induce a chemical reaction or cause combustion.
In a fire, heated containers are at risk of explosion. Its vapor is heavier than air,
which can spread to a relatively far place at a lower place. It will reignite when
exposed to open flames. Ethanol for industrial use is a central nervous system
inhibitor. Acute poisoning mostly occurs orally. Prolonged skin contact can
GB/T 6820-2016
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
ICS 71.080.60
G 17
Replacing GB/T 6820-1992
Ethanol for industrial use
ISSUED ON: DECEMBER 13, 2016
IMPLEMENTED ON: JULY 01, 2017
Issued by: General Administration of Quality Supervision, Inspection and
Quarantine of PRC;
Standardization Administration of PRC.
Table of Contents
Foreword ... 3
1 Scope ... 5
2 Normative references ... 5
3 Product categories ... 6
4 Requirements ... 6
5 Test methods ... 7
6 Inspection rules ... 13
7 Marking, packaging, storage and transportation ... 14
8 Safety ... 15
Appendix A (Normative) Typical gas chromatograms and retention times for the
determination of methanol, isopropanol, n-propanol, acetate, C4 + C5 alcohols
... 17
Ethanol for industrial use
1 Scope
This standard specifies the product classification, requirements, test methods,
inspection rules and signs, packaging, storage, transportation and safety of
ethanol for industrial use.
This standard applies to ethanol for industrial use.
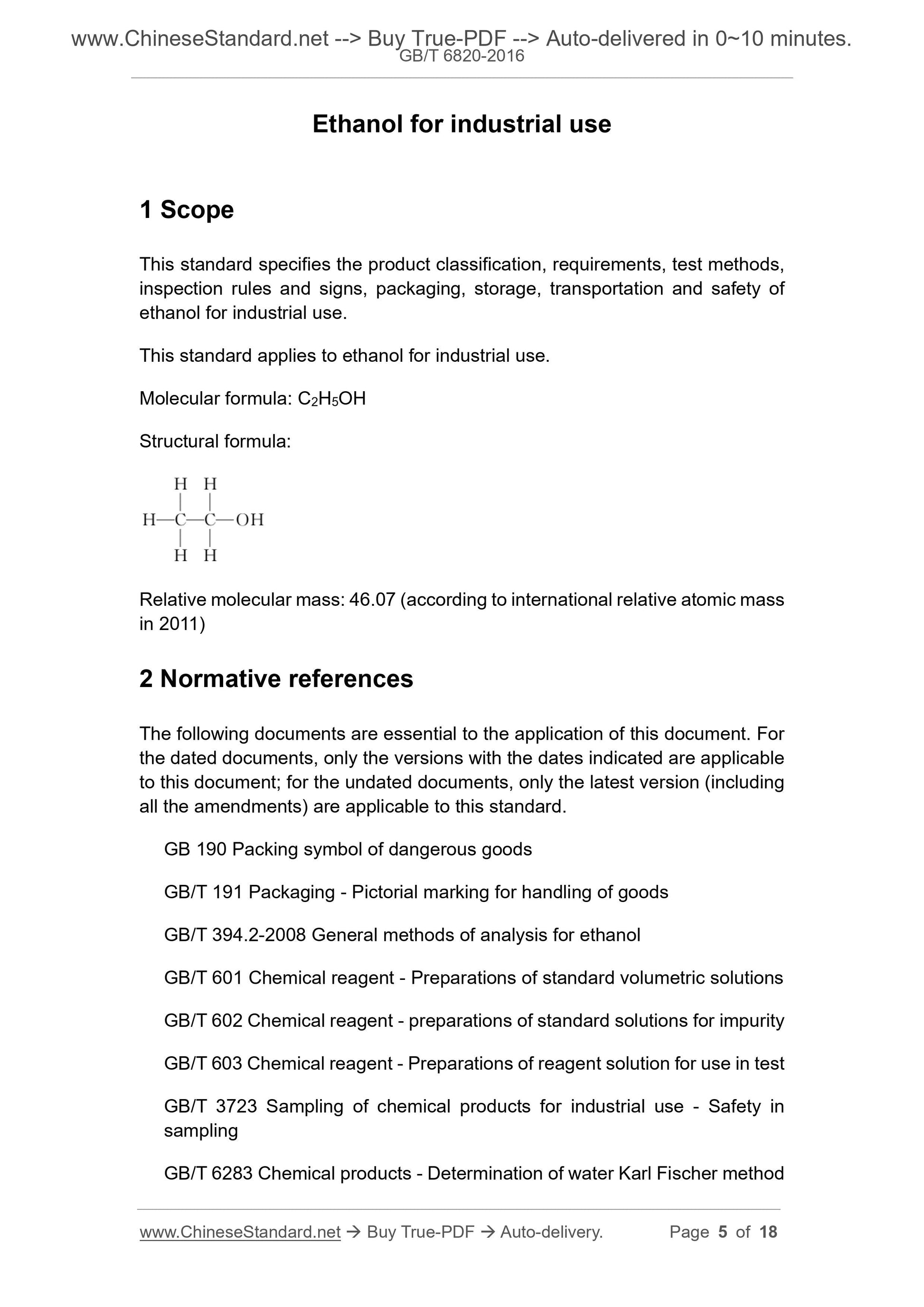
Molecular formula: C2H5OH
Structural formula:
Relative molecular mass: 46.07 (according to international relative atomic mass
in 2011)
2 Normative references
The following documents are essential to the application of this document. For
the dated documents, only the versions with the dates indicated are applicable
to this document; for the undated documents, only the latest version (including
all the amendments) are applicable to this standard.
GB 190 Packing symbol of dangerous goods
GB/T 191 Packaging - Pictorial marking for handling of goods
GB/T 394.2-2008 General methods of analysis for ethanol
GB/T 601 Chemical reagent - Preparations of standard volumetric solutions
GB/T 602 Chemical reagent - preparations of standard solutions for impurity
GB/T 603 Chemical reagent - Preparations of reagent solution for use in test
GB/T 3723 Sampling of chemical products for industrial use - Safety in
sampling
GB/T 6283 Chemical products - Determination of water Karl Fischer method
Determine it according to the method specified in Chapter 11 of GB/T 394.2-
2008.
5.7 Determination of aldehyde content
Determine it according to the method specified in Chapter 8 of GB/T 394.2-
2008. Use the iodine method as the arbitration method.
5.8 Determination of methanol, isopropanol, n-propanol, acetate, C4 + C5
alcohol content
5.8.1 Summary of method
Use gas chromatography, under the selected chromatographic conditions, to
separate the specimen by a chromatographic column. Use a hydrogen flame
ionization detector to detect it. Use the internal standard method, external
standard method, or corrected area normalization method to quantify it. Among
them, use the internal standard method as the arbitration method.
5.8.2 Reagents and solutions
5.8.2.1 Reference ethanol: 95% ethanol by volume, the limit of major impurities
is specified as follows: methanol is less than 2 mg/L; isopropanol is less than 2
mg/L; n-propanol is less than 2 mg/L; higher alcohol (C4 + C5 alcohol ) is less
than 1 mg/L; ester is less than 1 mg/L. When using this method for detection, it
shall not have impurity which may interfere with the internal standard.
5.8.2.2 Standard solution: Weigh 1 g of each of chromatographically pure
methanol, isopropanol, n-propanol, ethyl acetate, isopropyl acetate, 2-butanol,
iso-butanol, n-butanol, isoamyl alcohol, n-pentanol (accurate to 0.0001 g) in a
1000 mL volumetric flask. Use reference ethanol to make its volume reach to
the mark. After shaking it uniformly, it obtains the 1 g/L standard solution.
5.8.2.3 Internal standard solution: Weigh 1 g (accurate to 0.0001 g) of
chromatographic pure n-hexane in a 1000 mL volumetric flask. Use reference
ethanol to make its volume reach to the mark. After shaking it uniformly, it
obtains the 1 g/L internal standard solution.
5.8.2.4 Nitrogen: The volume fraction is not less than 99.95%; it is dried and
purified by silica gel and molecular sieve.
5.8.2.5 Hydrogen: The volume ...
Delivery: 9 seconds. Download (& Email) true-PDF + Invoice.
Get Quotation: Click GB/T 6820-2016 (Self-service in 1-minute)
Historical versions (Master-website): GB/T 6820-2016
Preview True-PDF (Reload/Scroll-down if blank)
GB/T 6820-2016
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
ICS 71.080.60
G 17
Replacing GB/T 6820-1992
Ethanol for industrial use
ISSUED ON: DECEMBER 13, 2016
IMPLEMENTED ON: JULY 01, 2017
Issued by: General Administration of Quality Supervision, Inspection and
Quarantine of PRC;
Standardization Administration of PRC.
Table of Contents
Foreword ... 3
1 Scope ... 5
2 Normative references ... 5
3 Product categories ... 6
4 Requirements ... 6
5 Test methods ... 7
6 Inspection rules ... 13
7 Marking, packaging, storage and transportation ... 14
8 Safety ... 15
Appendix A (Normative) Typical gas chromatograms and retention times for the
determination of methanol, isopropanol, n-propanol, acetate, C4 + C5 alcohols
... 17
Ethanol for industrial use
1 Scope
This standard specifies the product classification, requirements, test methods,
inspection rules and signs, packaging, storage, transportation and safety of
ethanol for industrial use.
This standard applies to ethanol for industrial use.
Molecular formula: C2H5OH
Structural formula:
Relative molecular mass: 46.07 (according to international relative atomic mass
in 2011)
2 Normative references
The following documents are essential to the application of this document. For
the dated documents, only the versions with the dates indicated are applicable
to this document; for the undated documents, only the latest version (including
all the amendments) are applicable to this standard.
GB 190 Packing symbol of dangerous goods
GB/T 191 Packaging - Pictorial marking for handling of goods
GB/T 394.2-2008 General methods of analysis for ethanol
GB/T 601 Chemical reagent - Preparations of standard volumetric solutions
GB/T 602 Chemical reagent - preparations of standard solutions for impurity
GB/T 603 Chemical reagent - Preparations of reagent solution for use in test
GB/T 3723 Sampling of chemical products for industrial use - Safety in
sampling
GB/T 6283 Chemical products - Determination of water Karl Fischer method
Determine it according to the method specified in Chapter 11 of GB/T 394.2-
2008.
5.7 Determination of aldehyde content
Determine it according to the method specified in Chapter 8 of GB/T 394.2-
2008. Use the iodine method as the arbitration method.
5.8 Determination of methanol, isopropanol, n-propanol, acetate, C4 + C5
alcohol content
5.8.1 Summary of method
Use gas chromatography, under the selected chromatographic conditions, to
separate the specimen by a chromatographic column. Use a hydrogen flame
ionization detector to detect it. Use the internal standard method, external
standard method, or corrected area normalization method to quantify it. Among
them, use the internal standard method as the arbitration method.
5.8.2 Reagents and solutions
5.8.2.1 Reference ethanol: 95% ethanol by volume, the limit of major impurities
is specified as follows: methanol is less than 2 mg/L; isopropanol is less than 2
mg/L; n-propanol is less than 2 mg/L; higher alcohol (C4 + C5 alcohol ) is less
than 1 mg/L; ester is less than 1 mg/L. When using this method for detection, it
shall not have impurity which may interfere with the internal standard.
5.8.2.2 Standard solution: Weigh 1 g of each of chromatographically pure
methanol, isopropanol, n-propanol, ethyl acetate, isopropyl acetate, 2-butanol,
iso-butanol, n-butanol, isoamyl alcohol, n-pentanol (accurate to 0.0001 g) in a
1000 mL volumetric flask. Use reference ethanol to make its volume reach to
the mark. After shaking it uniformly, it obtains the 1 g/L standard solution.
5.8.2.3 Internal standard solution: Weigh 1 g (accurate to 0.0001 g) of
chromatographic pure n-hexane in a 1000 mL volumetric flask. Use reference
ethanol to make its volume reach to the mark. After shaking it uniformly, it
obtains the 1 g/L internal standard solution.
5.8.2.4 Nitrogen: The volume fraction is not less than 99.95%; it is dried and
purified by silica gel and molecular sieve.
5.8.2.5 Hydrogen: The volume fraction is not less than 99.9%; it is dried and
purified by silica gel and molecular sieve.
5.8.2.6 Air: It is dried and purified by silica gel and molecular sieve.
5.8.3 Instruments and equipment
Take a small amount of the ethanol specimen to be tested in a 10 mL volumetric
flask. Accurately add 0.50 mL of the internal standard solution. Then use the
specimen to be tested to dilute it to the mark. Mix it uniformly. Inject 1 μL of
sample.
Use the retention time of the component peak and the internal standard peak
for qualitative purpose. Use the ratio of the peak area to calculate the content
of each component.
5.8.5.2 External standard method
5.8.5.2.1 Determination of correction factors
Accurately pipette 0.50 mL of the standard solution into a 10 mL volumetric flask.
Use the standard ethanol to dilute it to the mark. Mix it uniformly. Inject 1 μL of
sample. Use the peak area to calculate the correction factor f of each
component.
5.8.5.2.2 Determination of specimen
Under the same instrument conditions as the determination of the correction
factor, take a small amount of the ethanol specimen to be tested and accurately
inject 1 μL of sample.
Use the retention time of the component peak for qualitative purpose. Use the
peak area to calculate the content of each component.
5.8.5.3 Corrected area normalization method
5.8.5.3.1 Determination of correction factors
Accurately pipette 0.50 mL of standard solution into a 10 mL volumetric flask.
Use the standard ethanol to dilute it to the mark. Mix it uniformly. Accurately
inject 1 μL of sample. Use the peak area to calculate the correction factor f for
the relative ethanol of each component except ethanol.
5.8.5.3.2 Determination of specimen
Take a small amount of ethanol specimen to be tested and accurately inject 1
μL of specimen.
Use the retention time of the component peak for qualitative purpose. Use the
ratio of the peak area to calculate the content of each component.
5.8.6 Calculation of results
5.8.6.1 Internal standard method (arbitration method)
5.8.6.1.1 The relative correction factor fi for component i is calculated according
reporting result. The absolute value of the difference between the two parallel
determinations of all components shall meet the requirements of Table 3.
Table 3 -- Maximum allowable differences between two parallel
determinations of each component in the determination
Content of component More than or equal to 10 mg/L
Between 10 mg/L and 5
mg/L
Less than or equal to
5 mg/L
Maximum allowable difference X x 10% X x 20% X x 50%
5.9 Determination of oxidation time of potassium permanganate
Determine it according to the method as specified in Chapter 7 of GB/T 394.2-
2008.
5.10 Determination of evaporation residue
Determine it according to GB/T 6324.2.
Take the arithmetic mean of the two parallel determination results as the
determination result. The difference between the two parallel determination
results is not greater than 2 mg/L.
5.11 Determination of chroma in sulfuric acid test
Determine it according to the method specified in Chapter 6 of GB/T 394.2-
2008.
5.12 Water miscibility test
Determine it according to the method specified in GB/T 6324.1. Choose the
ratio of specimen and water miscibility at 1 + 9 and 1 + 19, respectively.
6 Inspection rules
6.1 Inspection is divided into exit-factory inspection and type inspection. The
appearance and the chromaticity, ethanol, acid, aldehyde, methanol,
isopropanol, n-propanol, acetate, C4 + C5 alcohol, oxidation time of potassium
permanganate and moisture in Table 1 are the exit-factory inspection items and
shall be inspected batch by batch. In the case of normal production, type
inspection shall be carried out at least once a month. Type inspection shall also
be carried out in one of the following cases:
a) In case of update of key production processes;
b) In case of change of the main raw materials;
c) In case of production restoration after suspension;
of the manufacturer. The manufacturer shall ensure that each batch of exit-
factory products meets the requirements of this standard and is accompanied
by a quality certificate in a certain format, which includes:
a) Product name;
b) Product model and grade;
c) Name and address of manufacturer;
d) Production date or batch number;
e) No. of this standard;
f) Industrial product production license marking and number.
7.2 Packaging
Ethanol for industrial use shall be stored in a clean, dry steel tanker or other
container that can guarantee quality.
7.3 Storage
Ethanol for industrial use shall be stored in a cool, dry, ventilated environment
which is isolated from heat sources and fires.
7.4 Transport
Ethanol for industrial use is a flammable and dangerous product. It must not be
exposed to sunlight during transportation; it shall not be stacked with flammable
materials and oxides. It shall strictly follow the relevant provisions for the
transportation of hazardous chemicals.
8 Safety
8.1 Warning of danger
Ethanol for industrial use is an irritating, flammable liquid. The melting point is -
114.3 °C; the boiling point is 78.4 °C; the flash point is 12 °C; the ignition
temperature is 363 °C. Its vapor and air may form explosive mixture; the
explosion limit is 3.3% (volume fraction) ~ 19.0% (volume fraction). When
exposed to open flame and high heat, it may cause combustion and explosion.
Contact with an oxidant may induce a chemical reaction or cause combustion.
In a fire, heated containers are at risk of explosion. Its vapor is heavier than air,
which can spread to a relatively far place at a lower place. It will reignite when
exposed to open flames. Ethanol for industrial use is a central nervous system
inhibitor. Acute poisoning mostly occurs orally. Prolonged skin contact can
GB/T 6820-2016
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
ICS 71.080.60
G 17
Replacing GB/T 6820-1992
Ethanol for industrial use
ISSUED ON: DECEMBER 13, 2016
IMPLEMENTED ON: JULY 01, 2017
Issued by: General Administration of Quality Supervision, Inspection and
Quarantine of PRC;
Standardization Administration of PRC.
Table of Contents
Foreword ... 3
1 Scope ... 5
2 Normative references ... 5
3 Product categories ... 6
4 Requirements ... 6
5 Test methods ... 7
6 Inspection rules ... 13
7 Marking, packaging, storage and transportation ... 14
8 Safety ... 15
Appendix A (Normative) Typical gas chromatograms and retention times for the
determination of methanol, isopropanol, n-propanol, acetate, C4 + C5 alcohols
... 17
Ethanol for industrial use
1 Scope
This standard specifies the product classification, requirements, test methods,
inspection rules and signs, packaging, storage, transportation and safety of
ethanol for industrial use.
This standard applies to ethanol for industrial use.
Molecular formula: C2H5OH
Structural formula:
Relative molecular mass: 46.07 (according to international relative atomic mass
in 2011)
2 Normative references
The following documents are essential to the application of this document. For
the dated documents, only the versions with the dates indicated are applicable
to this document; for the undated documents, only the latest version (including
all the amendments) are applicable to this standard.
GB 190 Packing symbol of dangerous goods
GB/T 191 Packaging - Pictorial marking for handling of goods
GB/T 394.2-2008 General methods of analysis for ethanol
GB/T 601 Chemical reagent - Preparations of standard volumetric solutions
GB/T 602 Chemical reagent - preparations of standard solutions for impurity
GB/T 603 Chemical reagent - Preparations of reagent solution for use in test
GB/T 3723 Sampling of chemical products for industrial use - Safety in
sampling
GB/T 6283 Chemical products - Determination of water Karl Fischer method
Determine it according to the method specified in Chapter 11 of GB/T 394.2-
2008.
5.7 Determination of aldehyde content
Determine it according to the method specified in Chapter 8 of GB/T 394.2-
2008. Use the iodine method as the arbitration method.
5.8 Determination of methanol, isopropanol, n-propanol, acetate, C4 + C5
alcohol content
5.8.1 Summary of method
Use gas chromatography, under the selected chromatographic conditions, to
separate the specimen by a chromatographic column. Use a hydrogen flame
ionization detector to detect it. Use the internal standard method, external
standard method, or corrected area normalization method to quantify it. Among
them, use the internal standard method as the arbitration method.
5.8.2 Reagents and solutions
5.8.2.1 Reference ethanol: 95% ethanol by volume, the limit of major impurities
is specified as follows: methanol is less than 2 mg/L; isopropanol is less than 2
mg/L; n-propanol is less than 2 mg/L; higher alcohol (C4 + C5 alcohol ) is less
than 1 mg/L; ester is less than 1 mg/L. When using this method for detection, it
shall not have impurity which may interfere with the internal standard.
5.8.2.2 Standard solution: Weigh 1 g of each of chromatographically pure
methanol, isopropanol, n-propanol, ethyl acetate, isopropyl acetate, 2-butanol,
iso-butanol, n-butanol, isoamyl alcohol, n-pentanol (accurate to 0.0001 g) in a
1000 mL volumetric flask. Use reference ethanol to make its volume reach to
the mark. After shaking it uniformly, it obtains the 1 g/L standard solution.
5.8.2.3 Internal standard solution: Weigh 1 g (accurate to 0.0001 g) of
chromatographic pure n-hexane in a 1000 mL volumetric flask. Use reference
ethanol to make its volume reach to the mark. After shaking it uniformly, it
obtains the 1 g/L internal standard solution.
5.8.2.4 Nitrogen: The volume fraction is not less than 99.95%; it is dried and
purified by silica gel and molecular sieve.
5.8.2.5 Hydrogen: The volume ...
Share