1
/
af
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY 0174-2019 English PDF (YY0174-2019)
YY 0174-2019 English PDF (YY0174-2019)
Normalpris
$230.00 USD
Normalpris
Udsalgspris
$230.00 USD
Stykpris
/
pr.
Levering beregnes ved betaling.
Tilgængelighed for afhentning kunne ikke indlæses
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY 0174-2019
Historical versions: YY 0174-2019
Preview True-PDF (Reload/Scroll if blank)
YY 0174-2019: [YY/T 0174-2019] Scalpel blade
YY 0174-2019
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.30
C 31
Replacing YY 0174-2005
Scalpel blade
ISSUED ON: MAY 31, 2019
IMPLEMENTED ON: JUNE 01, 2020
Issued by: National Medical Products Administration
Table of Contents
Foreword ... 3
1 Scope ... 5
2 Normative references ... 5
3 Classification and marks ... 6
4 Requirements ... 7
5 Test methods ... 8
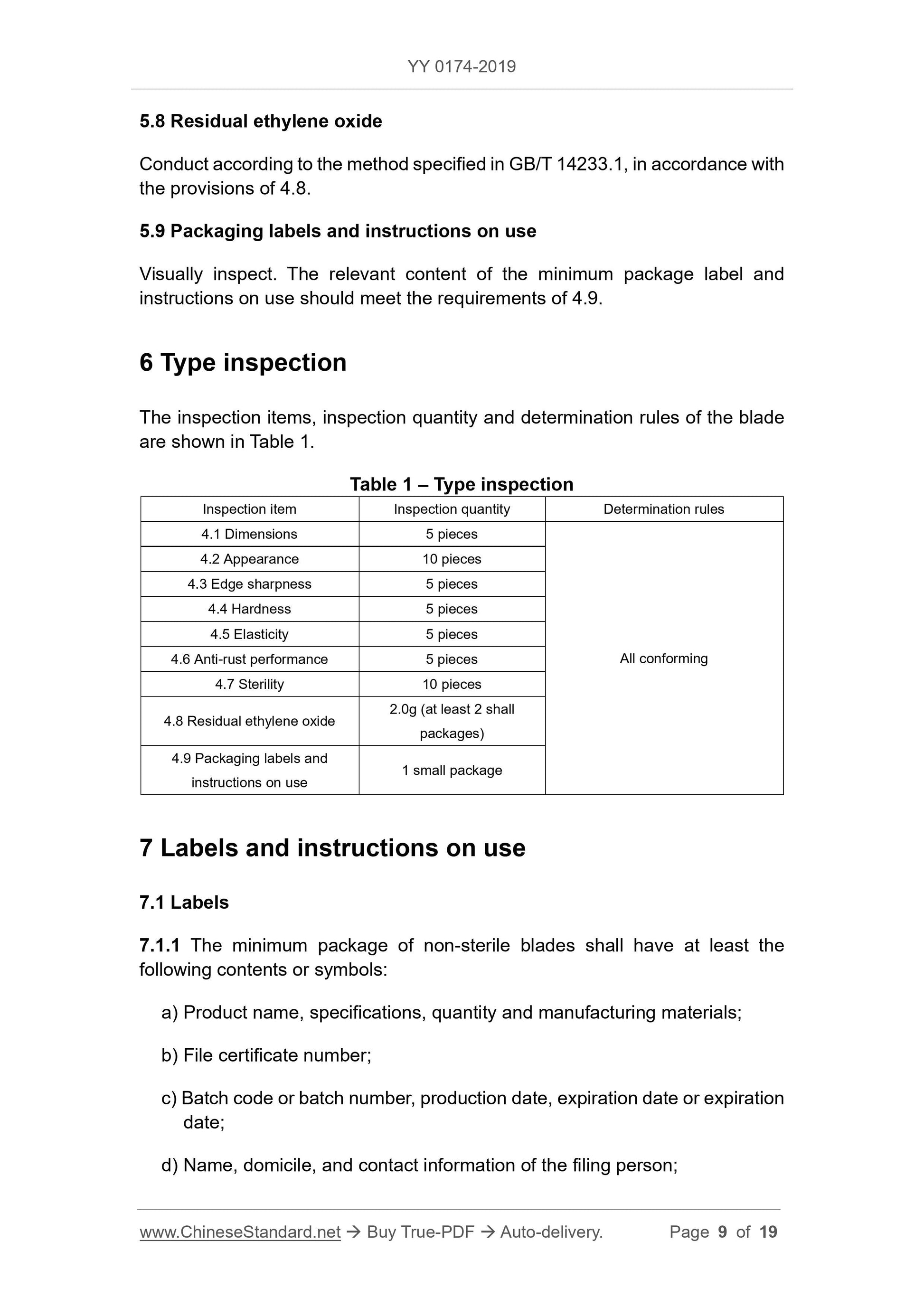
6 Type inspection ... 9
7 Labels and instructions on use ... 9
8 Packaging, transportation, storage and validity period ... 12
Annex A (informative) Blade specifications type... 13
Annex B (normative) Test method for scalpel blade edge sharpness ... 16
Annex C (normative) Test method for elasticity ... 18
Scalpel blade
1 Scope
This Standard specifies the classification and marks, requirements, test
methods, type inspection, labels and instructions on use, packaging,
transportation, storage and valid period of scalpel blade.
This Standard is applicable to the scalpel blade that is installed on the scalpel
handle for cutting soft tissue (hereinafter referred to as the blade).
2 Normative references
The following referenced documents are indispensable for the application of
this document. For dated references, only the edition cited applies. For undated
references, the latest edition of the referenced document (including any
amendments) applies.
GB/T 191, Packaging and storage marks
GB/T 1299, Alloy tool steels
GB/T 4340.1, Metallic materials - Vickers hardness test - Part 1: Test method
GB 8662, Fitting dimension between scalpel blades and handles
GB/T 9969, General Principles for Preparation of Instructions for Use of
Industrial Products
GB/T 14233.1, Test methods for infusion transfusion injection equipment for
medical use - Part 1: Chemical analysis methods
YY 0167-2005, Non-absorbable surgical suture
YY/T 0294.1, Surgical instruments - Metallic materials - Part 1: Stainless
steel
YY/T 0466.1, Medical devices - Symbols to be used with medical device
labels, labelling and information to be supplied - Part 1: General
requirements
YY/T 1052, Marking requirements for surgical instruments
Pharmacopoeia of the People's Republic of China (Edition 2015· Fourth
4 Requirements
4.1 dimensions
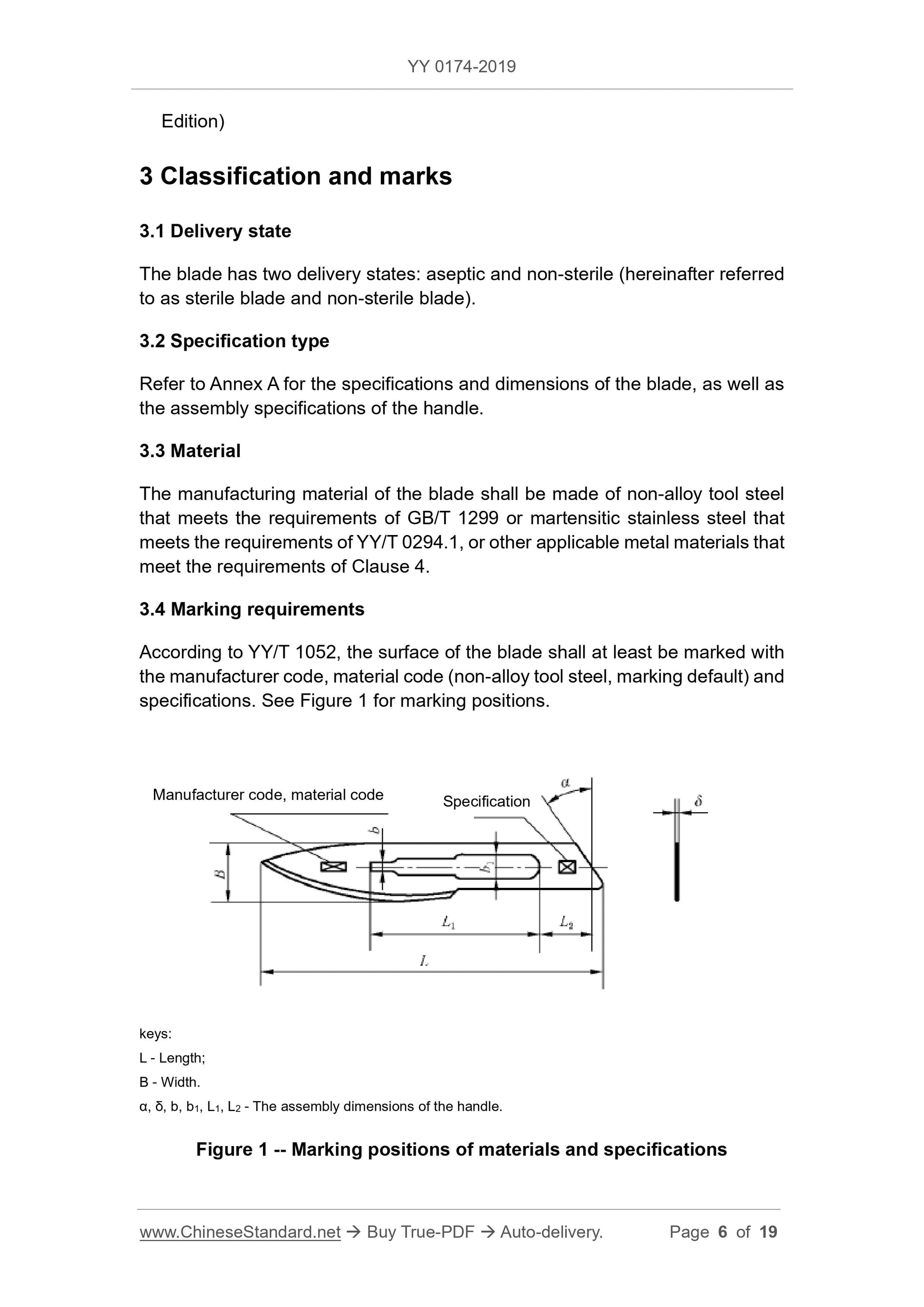
The blade assembly dimensions α, δ, b, b1, L1 and L2, see Figure 1, shall comply
with the provisions of GB 8662.
4.2 Appearance
4.2.1 The value of the blade surface roughness Ra shall not be greater than
0.4μm. The value of the surface roughness Ra of the cutting-edge surface shall
not be greater than 0.8μm.
4.2.2 The blade edge shall be free of nicks, white edges, curls, cracks and so
on.
4.2.3 The width of both sides of the cutting edge of the blade shall be similar,
without focal spots on the surface.
4.2.4 The blade shall be flat, without cracks, sharp edges, burrs, obvious pits.
4.3 Edge sharpness
The blade edge shall be sharp. The cutting force shall not be greater than 0.80N.
4.4 Hardness
The blade shall be heat treated. Its hardness is not less than 650HV10.
4.5 Elasticity
The blade shall have good elasticity. After the test, there is no fracture and no
obvious deformation.
4.6 Anti-rust performance
The blade shall have good rust resistance. There shall be no rust spots on the
surface.
4.7 Sterility
If it is a sterile blade, it shall be sterilized through a confirmed sterilization
process. The blade shall be sterile.
4.8 Ethylene oxide residue
If the blade is sterilized with ethylene oxide gas, the residual amount of ethylene
oxide shall not exceed 10μg/g.
e) Manufacturer’s name, domicile, production address, contact information
and production file certificate number;
f) Words or symbols of "Other contents are detailed in the instructions on
use".
7.1.2 The minimum package of sterile blades shall have at least the following
contents or symbols:
a) Product name and specifications;
b) Sterilization method, sterility, single-use mark;
c) Product registration certificate number;
d) Words or symbols of "Do not use if the package is damaged";
e) Batch code or batch number, production date, expiration date or expiration
date;
f) Name and domicile of the registrant or manufacturer;
g) Words or symbols of "Other contents are detailed in the instructions on
use".
7.1.3 The minimum sales unit packaging shall have at least the following
contents or symbols:
a) Product name;
b) Specifications, quantity;
c) Sterilization method (for sterile blades), single-use mark;
d) Batch code or batch number, production date, expiration date or expiration
date;
e) Product registration certificate number or record certificate number,
production license number or production record certificate number;
f) Name of the registrant or filing person, the name of the manufacturer, the
address, and the contact information.
7.1.4 There shall be an inspection certificate in each minimum sales unit. At
least the following content shall be included on the certificate:
a) Name of the registrant or filing person or manufacturer;
b) Number of product technical requirements;
c) Batch code or batch number;
d) Inspection date and inspector code.
7.1.5 The packaging, storage and transportation marks on the package shall
comply with the relevant regulations of GB/T 191 and YY/T 0466.1.
7.2 Instructions on use
There shall be instructions on use in each minimum sales unit. The preparation
of the instructions on use shall comply with the requirements of GB/T 9969, at
least containing the followings:
a) Product name, specifications;
b) Name, address, contact information and after-sales service information of
the registrant or filing person or manufacturer; If it is entrusted production,
it shall also indicate the name, address and production license number of
the entrusted production enterprise or production record document
number;
c) Product registration certificate number or record certificate number,
production license number or production record certificate number;
d) Number of product technical requirements;
e) Product performance, manufacturing materials, scope of application;
f) Installation and use instructions or icons;
g) Storage conditions and methods;
h) Description on one-time use, detailed sterilization or disinfection method,
production date, use period or expiration date;
i) Explanation of the graphics, symbols, abbreviations used in the label;
j) Preparation of instructions on use or revision date;
k) Contraindications, precautions, warnings and reminders:
1) Requirements to ensure the correct and safe use of the blade, and post-
processing requirements for safe use;
2) Precautions when the blade is used in conjunction with other
instruments;
3) The sterile blade shall indicate the treatment method when the
minimum single package is damaged.
Get QUOTATION in 1-minute: Click YY 0174-2019
Historical versions: YY 0174-2019
Preview True-PDF (Reload/Scroll if blank)
YY 0174-2019: [YY/T 0174-2019] Scalpel blade
YY 0174-2019
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.30
C 31
Replacing YY 0174-2005
Scalpel blade
ISSUED ON: MAY 31, 2019
IMPLEMENTED ON: JUNE 01, 2020
Issued by: National Medical Products Administration
Table of Contents
Foreword ... 3
1 Scope ... 5
2 Normative references ... 5
3 Classification and marks ... 6
4 Requirements ... 7
5 Test methods ... 8
6 Type inspection ... 9
7 Labels and instructions on use ... 9
8 Packaging, transportation, storage and validity period ... 12
Annex A (informative) Blade specifications type... 13
Annex B (normative) Test method for scalpel blade edge sharpness ... 16
Annex C (normative) Test method for elasticity ... 18
Scalpel blade
1 Scope
This Standard specifies the classification and marks, requirements, test
methods, type inspection, labels and instructions on use, packaging,
transportation, storage and valid period of scalpel blade.
This Standard is applicable to the scalpel blade that is installed on the scalpel
handle for cutting soft tissue (hereinafter referred to as the blade).
2 Normative references
The following referenced documents are indispensable for the application of
this document. For dated references, only the edition cited applies. For undated
references, the latest edition of the referenced document (including any
amendments) applies.
GB/T 191, Packaging and storage marks
GB/T 1299, Alloy tool steels
GB/T 4340.1, Metallic materials - Vickers hardness test - Part 1: Test method
GB 8662, Fitting dimension between scalpel blades and handles
GB/T 9969, General Principles for Preparation of Instructions for Use of
Industrial Products
GB/T 14233.1, Test methods for infusion transfusion injection equipment for
medical use - Part 1: Chemical analysis methods
YY 0167-2005, Non-absorbable surgical suture
YY/T 0294.1, Surgical instruments - Metallic materials - Part 1: Stainless
steel
YY/T 0466.1, Medical devices - Symbols to be used with medical device
labels, labelling and information to be supplied - Part 1: General
requirements
YY/T 1052, Marking requirements for surgical instruments
Pharmacopoeia of the People's Republic of China (Edition 2015· Fourth
4 Requirements
4.1 dimensions
The blade assembly dimensions α, δ, b, b1, L1 and L2, see Figure 1, shall comply
with the provisions of GB 8662.
4.2 Appearance
4.2.1 The value of the blade surface roughness Ra shall not be greater than
0.4μm. The value of the surface roughness Ra of the cutting-edge surface shall
not be greater than 0.8μm.
4.2.2 The blade edge shall be free of nicks, white edges, curls, cracks and so
on.
4.2.3 The width of both sides of the cutting edge of the blade shall be similar,
without focal spots on the surface.
4.2.4 The blade shall be flat, without cracks, sharp edges, burrs, obvious pits.
4.3 Edge sharpness
The blade edge shall be sharp. The cutting force shall not be greater than 0.80N.
4.4 Hardness
The blade shall be heat treated. Its hardness is not less than 650HV10.
4.5 Elasticity
The blade shall have good elasticity. After the test, there is no fracture and no
obvious deformation.
4.6 Anti-rust performance
The blade shall have good rust resistance. There shall be no rust spots on the
surface.
4.7 Sterility
If it is a sterile blade, it shall be sterilized through a confirmed sterilization
process. The blade shall be sterile.
4.8 Ethylene oxide residue
If the blade is sterilized with ethylene oxide gas, the residual amount of ethylene
oxide shall not exceed 10μg/g.
e) Manufacturer’s name, domicile, production address, contact information
and production file certificate number;
f) Words or symbols of "Other contents are detailed in the instructions on
use".
7.1.2 The minimum package of sterile blades shall have at least the following
contents or symbols:
a) Product name and specifications;
b) Sterilization method, sterility, single-use mark;
c) Product registration certificate number;
d) Words or symbols of "Do not use if the package is damaged";
e) Batch code or batch number, production date, expiration date or expiration
date;
f) Name and domicile of the registrant or manufacturer;
g) Words or symbols of "Other contents are detailed in the instructions on
use".
7.1.3 The minimum sales unit packaging shall have at least the following
contents or symbols:
a) Product name;
b) Specifications, quantity;
c) Sterilization method (for sterile blades), single-use mark;
d) Batch code or batch number, production date, expiration date or expiration
date;
e) Product registration certificate number or record certificate number,
production license number or production record certificate number;
f) Name of the registrant or filing person, the name of the manufacturer, the
address, and the contact information.
7.1.4 There shall be an inspection certificate in each minimum sales unit. At
least the following content shall be included on the certificate:
a) Name of the registrant or filing person or manufacturer;
b) Number of product technical requirements;
c) Batch code or batch number;
d) Inspection date and inspector code.
7.1.5 The packaging, storage and transportation marks on the package shall
comply with the relevant regulations of GB/T 191 and YY/T 0466.1.
7.2 Instructions on use
There shall be instructions on use in each minimum sales unit. The preparation
of the instructions on use shall comply with the requirements of GB/T 9969, at
least containing the followings:
a) Product name, specifications;
b) Name, address, contact information and after-sales service information of
the registrant or filing person or manufacturer; If it is entrusted production,
it shall also indicate the name, address and production license number of
the entrusted production enterprise or production record document
number;
c) Product registration certificate number or record certificate number,
production license number or production record certificate number;
d) Number of product technical requirements;
e) Product performance, manufacturing materials, scope of application;
f) Installation and use instructions or icons;
g) Storage conditions and methods;
h) Description on one-time use, detailed sterilization or disinfection method,
production date, use period or expiration date;
i) Explanation of the graphics, symbols, abbreviations used in the label;
j) Preparation of instructions on use or revision date;
k) Contraindications, precautions, warnings and reminders:
1) Requirements to ensure the correct and safe use of the blade, and post-
processing requirements for safe use;
2) Precautions when the blade is used in conjunction with other
instruments;
3) The sterile blade shall indicate the treatment method when the
minimum single package is damaged.
Share