1
/
von
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 0282-2009 English PDF (YYT0282-2009)
YY/T 0282-2009 English PDF (YYT0282-2009)
Normaler Preis
$150.00 USD
Normaler Preis
Verkaufspreis
$150.00 USD
Grundpreis
/
pro
Versand wird beim Checkout berechnet
Verfügbarkeit für Abholungen konnte nicht geladen werden
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY/T 0282-2009
Historical versions: YY/T 0282-2009
Preview True-PDF (Reload/Scroll if blank)
YY/T 0282-2009: Syringe needle
YY/T 0282-2009
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.20
C 31
Replacing YY/T 0282-1995, YY 91019-1999,
YY 91020-1999, YY/T 91140-1999
Syringe needle
注射针
ISSUED ON. JUNE 16, 2009
IMPLEMENTED ON. OCTOBER 1, 2010
Issued by. China Food and Drug Administration
Table of Contents
Foreword ... 3
Introduction ... 4
1 Scope ... 5
2 Normative references ... 5
3 Classification and marks... 5
4 Materials ... 7
5 Requirements ... 7
6 Marks, instructions for use ... 10
7 Packaging ... 11
8 Transport and storage ... 11
Annex A (Informative) Guidelines for syringe needle use ... 13
Annex B (Informative) Needle tip geometry and naming marks ... 14
Annex C (Informative) Needle puncture force and test methods... 15
Annex D (Normative) Type inspection rules ... 18
Foreword
This Standard replaces YY/T 0282-1995 Syringe needles, YY 91018-1999
Anaesthesia needles, YY 91019-1999 Dental needles, YY 91020-1999
Needles used for transfusion and infusion, and YY/T 91140-1999 Blood
transfusion needles.
Compared with YY/T 0282-1995, the main changes in this Standard are as
follows.
- expanded product specifications (Table 1 in 3.1 of YY/T 0282-1995,
Table 1 in 3.2 of this Standard);
- modified needle rigidities, toughness and corrosion resistance
requirements and test methods (4.6, 4.7, 4.7 and 5.2.4, 5.3.3, 5.3.4 of
Edition 1995, 5.2.1, 5.2.2, 5.2.3 of this Edition);
- modified needle sharpness requirements and test methods (4.10 and
5.3.6 of Edition 1995, 5.3.2 of this Edition).
Annex D of this Standard is normative. Annex A, Annex B and Annex C are
informative.
This Standard was proposed by China Food and Drug Administration.
This Standard shall be under the jurisdiction of National Technical Committee
on Medical Syringes (Needles) of Standardization Administration of China.
Main drafting organization of this Standard. Shanghai Esaye Medical
Apparatus Plastic Products Co., Ltd.
Main drafters of this Standard. Cao Xianming, Zhang Jinsheng.
This standard was issued in 1965, with the first revision in 1980, the second
revision in 1988, the third revision in 1995, the fourth revision in 2009.
Introduction
The " Syringe needle" standard has been implemented in China for nearly 40
years, playing an important guiding role in this field.
While YY/T 0282-1995 Syringe needles was revised, YY 91018-1999
Anaesthesia needles, YY 91019-1999 Dental needles, YY 91020-1999
Needles used for transfusion and infusion, and YY/T 91140-1999 Blood
transfusion needles were used as references. Its product structure, technical
performance requirements are the same, but because of the different parts
used, its needle diameter and length are of different sizes. And it also refers
to JIST 3101 "Syringe needle", the Japanese standard, which also included in
the above-mentioned standard products. Therefore, this revision shall merge
the above five standards into one. At the same time, for the current
production of " BCG (Bacille Calmette-Guerin) vaccine" needles, in view of
the same reasons above, is also incorporated in needle standards.
Syringe needle
1 Scope
This Standard specifies the requirements for injection liquid, vaccine,
anesthetic or intravenous infusion, blood transfusion needles with nominal
diameters of 0.4mm ~ 1.6mm, used for human body subcutaneous,
intradermal, muscle, oral and other parts.
2 Normative references
The provisions in following documents become the provisions of this
Standard through reference in this Standard. For dated references, the
subsequent amendments (excluding corrigendum) or revisions do not apply
to this Standard, however, parties who reach an agreement based on this
Standard are encouraged to study if the latest versions of these documents
are applicable. For undated references, the latest edition of the referenced
document applies.
GB/T 191, Packaging and storage marks
GB/T 1962.1, Conical fittings with a 6% (Luer) taper for syringes, needles
and certain other medical equipment - Part 1. General requirement
GB/T 1962.2, Conical fittings with a 6% (Luer) taper for syringes, needles
and certain other medical equipment - Part 2. Lock fittings
GB/T 18457, Stainless steel needle tubing for the manufacture of medical
devices
3 Classification and marks
3.1 Structure type
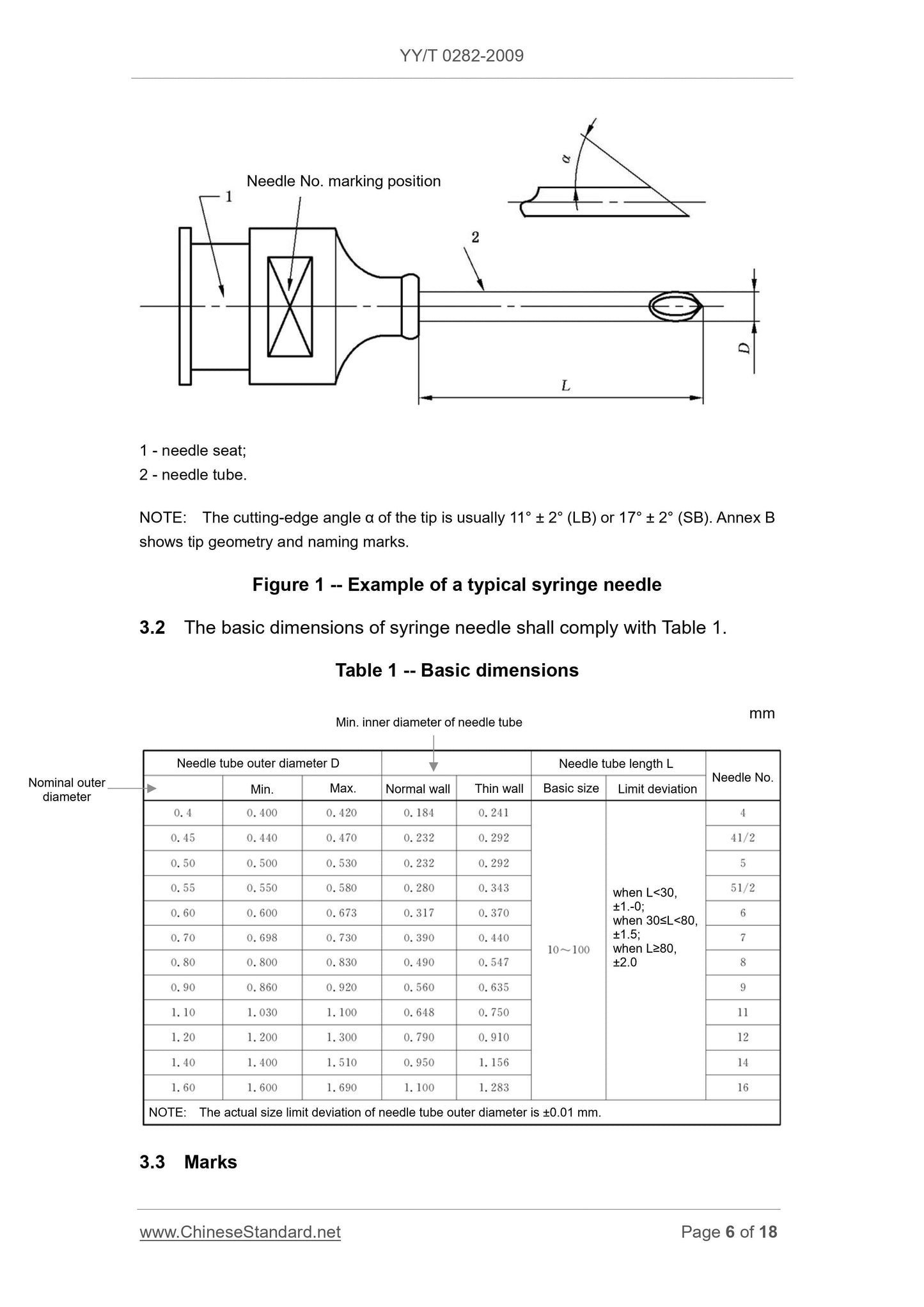
The structure type and part name of syringe needle are shown in Figure 1.
1 - needle seat;
2 - needle tube.
NOTE. The cutting-edge angle α of the tip is usually 11° ± 2° (LB) or 17° ± 2° (SB). Annex B
shows tip geometry and naming marks.
Figure 1 -- Example of a typical syringe needle
3.2 The basic dimensions of syringe needle shall comply with Table 1.
Table 1 -- Basic dimensions
mm
3.3 Marks
Needle tube length L
Needle No.Nominal outer
diameter Min. Max.
Needle No. marking position
Normal wall
Needle tube outer diameter D
Min. inner diameter of needle tube
Thin wall Basic size Limit deviation
NOTE. The actual size limit deviation of needle tube outer diameter is ±0.01 mm.
when L< 30,
±1.-0;
when 30≤L< 80,
±1.5;
when L≥80,
±2.0
The specifications of syringe needle product are represented in nominal
diameter of needle × needle tube length and wall type and edge angle. The
needle tube sizes are in mm.
Tube wall type. normal wall in RW, thin wall in TW
Edge angle. long bevel angle in LB, short bevel angle in SB
Marking example.
A syringe needle with tube nominal outer diameter of 0.7 mm, tube length of
32 mm, short bevel angle, thin wall is marked as.
0.7 × 32 TW SB
NOTE. When tube wall is normal and edge angle is long bevel angle, it shall not be marked.
4 Materials
The materials used to make syringe needle shall meet the requirements in
Clause 5.
5 Requirements
5.1 Needle seat
5.1.1 Needle seat conical connector shall be consistent with requirements
of GB/T1962.1 or GB/T1962.2.
5.1.2 With normal or corrected vision observation, the outer surface of the
needle seat shall be smooth; there shall be no cracks, defects; the coating
shall not fall off; the mark shall be clear.
5.2 Needle tube
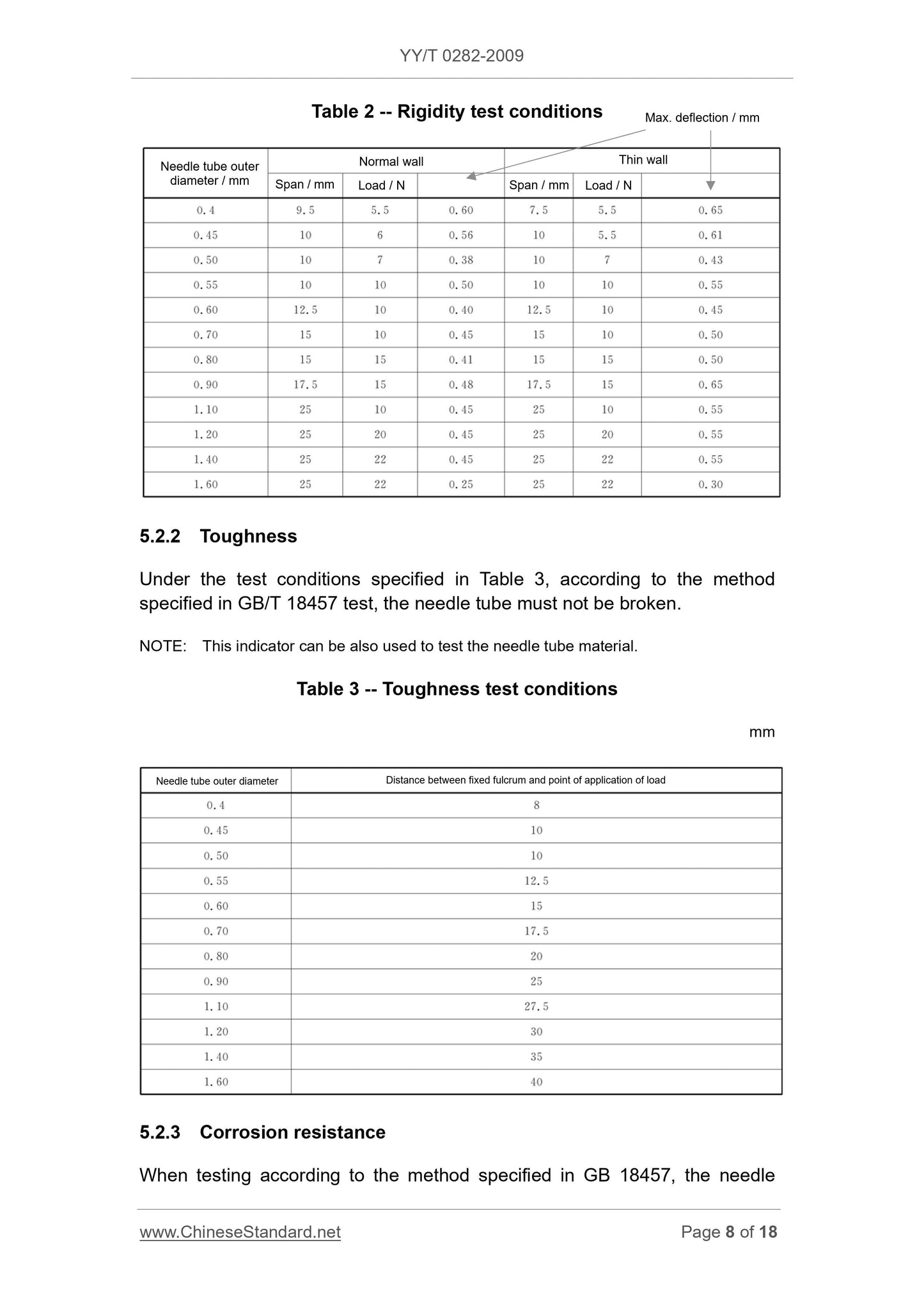
5.2.1 Rigidity
When testing according to the method specified in GB/T 18457, the needle
deflection value shall not exceed the provisions of Table 2.
NOTE. Needle material can also be tested if necessary.
Table 2 -- Rigidity test conditions
5.2.2 Toughness
Under the test conditions specified in Table 3, according to the method
specified in GB/T 18457 test, the needle tube must not be broken.
NOTE. This indicator can be also used to test the needle tube material.
Table 3 -- Toughness test conditions
mm
5.2.3 Corrosion resistance
When testing according to the method specified in GB 18457, the needle
Span / mm Span / mm
Needle tube outer
diameter / mm Load / N
Normal wall
Load / N
Thin wall
Max. deflection / mm
Needle tube outer diameter Distance between fixed fulcrum and point of application of load
soaked parts shall not have corrosion marks.
NOTE. This indicator can be also used to test the needle tube material.
5.2.4 With normal or corrected vision observation, the outer surface of the
needle tube shall be smooth, defect-free and free from impurities in the
metalworking process.
5.2.5 Needle tube bore shall be clean. Use a clean glass syringe to inject
glycerin and ethanol ...
Get QUOTATION in 1-minute: Click YY/T 0282-2009
Historical versions: YY/T 0282-2009
Preview True-PDF (Reload/Scroll if blank)
YY/T 0282-2009: Syringe needle
YY/T 0282-2009
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.20
C 31
Replacing YY/T 0282-1995, YY 91019-1999,
YY 91020-1999, YY/T 91140-1999
Syringe needle
注射针
ISSUED ON. JUNE 16, 2009
IMPLEMENTED ON. OCTOBER 1, 2010
Issued by. China Food and Drug Administration
Table of Contents
Foreword ... 3
Introduction ... 4
1 Scope ... 5
2 Normative references ... 5
3 Classification and marks... 5
4 Materials ... 7
5 Requirements ... 7
6 Marks, instructions for use ... 10
7 Packaging ... 11
8 Transport and storage ... 11
Annex A (Informative) Guidelines for syringe needle use ... 13
Annex B (Informative) Needle tip geometry and naming marks ... 14
Annex C (Informative) Needle puncture force and test methods... 15
Annex D (Normative) Type inspection rules ... 18
Foreword
This Standard replaces YY/T 0282-1995 Syringe needles, YY 91018-1999
Anaesthesia needles, YY 91019-1999 Dental needles, YY 91020-1999
Needles used for transfusion and infusion, and YY/T 91140-1999 Blood
transfusion needles.
Compared with YY/T 0282-1995, the main changes in this Standard are as
follows.
- expanded product specifications (Table 1 in 3.1 of YY/T 0282-1995,
Table 1 in 3.2 of this Standard);
- modified needle rigidities, toughness and corrosion resistance
requirements and test methods (4.6, 4.7, 4.7 and 5.2.4, 5.3.3, 5.3.4 of
Edition 1995, 5.2.1, 5.2.2, 5.2.3 of this Edition);
- modified needle sharpness requirements and test methods (4.10 and
5.3.6 of Edition 1995, 5.3.2 of this Edition).
Annex D of this Standard is normative. Annex A, Annex B and Annex C are
informative.
This Standard was proposed by China Food and Drug Administration.
This Standard shall be under the jurisdiction of National Technical Committee
on Medical Syringes (Needles) of Standardization Administration of China.
Main drafting organization of this Standard. Shanghai Esaye Medical
Apparatus Plastic Products Co., Ltd.
Main drafters of this Standard. Cao Xianming, Zhang Jinsheng.
This standard was issued in 1965, with the first revision in 1980, the second
revision in 1988, the third revision in 1995, the fourth revision in 2009.
Introduction
The " Syringe needle" standard has been implemented in China for nearly 40
years, playing an important guiding role in this field.
While YY/T 0282-1995 Syringe needles was revised, YY 91018-1999
Anaesthesia needles, YY 91019-1999 Dental needles, YY 91020-1999
Needles used for transfusion and infusion, and YY/T 91140-1999 Blood
transfusion needles were used as references. Its product structure, technical
performance requirements are the same, but because of the different parts
used, its needle diameter and length are of different sizes. And it also refers
to JIST 3101 "Syringe needle", the Japanese standard, which also included in
the above-mentioned standard products. Therefore, this revision shall merge
the above five standards into one. At the same time, for the current
production of " BCG (Bacille Calmette-Guerin) vaccine" needles, in view of
the same reasons above, is also incorporated in needle standards.
Syringe needle
1 Scope
This Standard specifies the requirements for injection liquid, vaccine,
anesthetic or intravenous infusion, blood transfusion needles with nominal
diameters of 0.4mm ~ 1.6mm, used for human body subcutaneous,
intradermal, muscle, oral and other parts.
2 Normative references
The provisions in following documents become the provisions of this
Standard through reference in this Standard. For dated references, the
subsequent amendments (excluding corrigendum) or revisions do not apply
to this Standard, however, parties who reach an agreement based on this
Standard are encouraged to study if the latest versions of these documents
are applicable. For undated references, the latest edition of the referenced
document applies.
GB/T 191, Packaging and storage marks
GB/T 1962.1, Conical fittings with a 6% (Luer) taper for syringes, needles
and certain other medical equipment - Part 1. General requirement
GB/T 1962.2, Conical fittings with a 6% (Luer) taper for syringes, needles
and certain other medical equipment - Part 2. Lock fittings
GB/T 18457, Stainless steel needle tubing for the manufacture of medical
devices
3 Classification and marks
3.1 Structure type
The structure type and part name of syringe needle are shown in Figure 1.
1 - needle seat;
2 - needle tube.
NOTE. The cutting-edge angle α of the tip is usually 11° ± 2° (LB) or 17° ± 2° (SB). Annex B
shows tip geometry and naming marks.
Figure 1 -- Example of a typical syringe needle
3.2 The basic dimensions of syringe needle shall comply with Table 1.
Table 1 -- Basic dimensions
mm
3.3 Marks
Needle tube length L
Needle No.Nominal outer
diameter Min. Max.
Needle No. marking position
Normal wall
Needle tube outer diameter D
Min. inner diameter of needle tube
Thin wall Basic size Limit deviation
NOTE. The actual size limit deviation of needle tube outer diameter is ±0.01 mm.
when L< 30,
±1.-0;
when 30≤L< 80,
±1.5;
when L≥80,
±2.0
The specifications of syringe needle product are represented in nominal
diameter of needle × needle tube length and wall type and edge angle. The
needle tube sizes are in mm.
Tube wall type. normal wall in RW, thin wall in TW
Edge angle. long bevel angle in LB, short bevel angle in SB
Marking example.
A syringe needle with tube nominal outer diameter of 0.7 mm, tube length of
32 mm, short bevel angle, thin wall is marked as.
0.7 × 32 TW SB
NOTE. When tube wall is normal and edge angle is long bevel angle, it shall not be marked.
4 Materials
The materials used to make syringe needle shall meet the requirements in
Clause 5.
5 Requirements
5.1 Needle seat
5.1.1 Needle seat conical connector shall be consistent with requirements
of GB/T1962.1 or GB/T1962.2.
5.1.2 With normal or corrected vision observation, the outer surface of the
needle seat shall be smooth; there shall be no cracks, defects; the coating
shall not fall off; the mark shall be clear.
5.2 Needle tube
5.2.1 Rigidity
When testing according to the method specified in GB/T 18457, the needle
deflection value shall not exceed the provisions of Table 2.
NOTE. Needle material can also be tested if necessary.
Table 2 -- Rigidity test conditions
5.2.2 Toughness
Under the test conditions specified in Table 3, according to the method
specified in GB/T 18457 test, the needle tube must not be broken.
NOTE. This indicator can be also used to test the needle tube material.
Table 3 -- Toughness test conditions
mm
5.2.3 Corrosion resistance
When testing according to the method specified in GB 18457, the needle
Span / mm Span / mm
Needle tube outer
diameter / mm Load / N
Normal wall
Load / N
Thin wall
Max. deflection / mm
Needle tube outer diameter Distance between fixed fulcrum and point of application of load
soaked parts shall not have corrosion marks.
NOTE. This indicator can be also used to test the needle tube material.
5.2.4 With normal or corrected vision observation, the outer surface of the
needle tube shall be smooth, defect-free and free from impurities in the
metalworking process.
5.2.5 Needle tube bore shall be clean. Use a clean glass syringe to inject
glycerin and ethanol ...
Share