1
/
von
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 0506.5-2009 English PDF (YYT0506.5-2009)
YY/T 0506.5-2009 English PDF (YYT0506.5-2009)
Normaler Preis
$190.00 USD
Normaler Preis
Verkaufspreis
$190.00 USD
Grundpreis
/
pro
Versand wird beim Checkout berechnet
Verfügbarkeit für Abholungen konnte nicht geladen werden
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY/T 0506.5-2009
Historical versions: YY/T 0506.5-2009
Preview True-PDF (Reload/Scroll if blank)
YY/T 0506.5-2009: Surgical drapes, gowns and clean air suits for patients, clinical staff and equipment. Part 5: Test method for resistance to dry microbial penetration
YY/T 0506.5-2009
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.140
C 46
Surgical drapes, gowns and clean air suits for
patients, clinical staff and equipment - Part 5: Test
method for resistance to dry microbial penetration
(ISO 22612:2005, MOD)
ISSUED ON: JUNE 16, 2009
IMPLEMENTED ON: DECEMBER 01, 2010
Issued by: China Food and Drug Administration
Table of Contents
Foreword ... 3
Introduction ... 4
1 Scope ... 5
2 Normative references ... 5
3 Terms and definitions ... 5
4 Principle ... 5
5 Test conditions ... 6
6 Equipment ... 6
7 Procedure ... 10
8 Test report ... 11
Appendix A (Informative) TGE agar medium ... 12
Appendix NA (Informative) Technical differences between this Standard and
ISO 22612:2005 and their reasons ... 13
References ... 14
Surgical drapes, gowns and clean air suits for
patients, clinical staff and equipment - Part 5: Test
method for resistance to dry microbial penetration
1 Scope
This part of YY/T 0506 stipulates the test method that is used to evaluate the
resistance of permeability to bacterial particles of barrier materials.
2 Normative references
The terms in the following documents become the terms of this part by
reference to this part of YY/T 0506. For dated references, all subsequent
amendments (not including errata content) or revisions do not apply to this part.
However, parties to agreements that are based on this part are encouraged to
study whether the latest versions of these documents can be used. For undated
references, the latest edition applies to this part.
YY/T 0506.1, Surgical drapes, gowns and clean air suits for patients, clinical
staff and equipment - Part 1: General requirements for manufacturers,
processors and products
3 Terms and definitions
The terms and definitions of YY/T 0506.1 apply to this part of YY/T 0506.
4 Principle
This test is carried out on test pieces that are respectively fixed on a container.
Among these containers, 5 containers that carry bacillus subtilis talc powder
and 1 container that is added with untainted talc powder are used as controls.
Insert a petri dish at the bottom of each container that is close to the bottom of
the test piece.
The equipment that supports the container is oscillated by a gas ball oscillator;
all the talc powders that penetrate the test piece fall onto the petri dish; take out
the petri dish and cultivate.
Count the growing bacterial colonies.
5 Test conditions
Adjust the state and test the samples at 20 °C ± 2 °C and the relative humidity
of 65% ± 5%.
6 Equipment
6.1 General structure
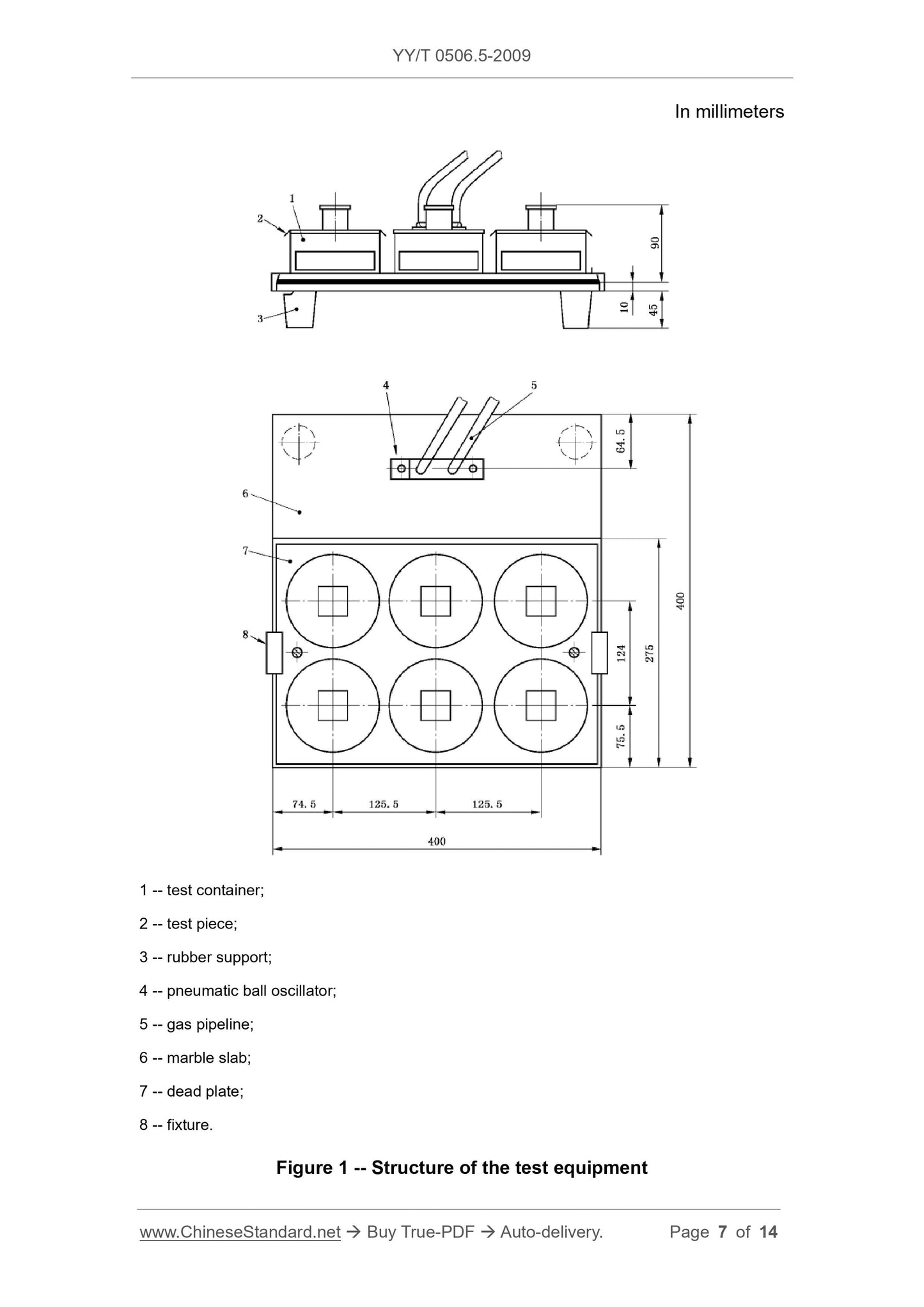
Note: see Figure 1.
6.1.1 10 mm thick stone slab, such as marble, 40 cm × 40 cm, with 4 rubber
supports at each corner.
6.1.2 Pneumatic ball oscillator1, that can produce 20800 vibrations per minute;
the force is 650 N.
6.1.3 An oscillator that is connected to one side of the upper surface of the
marble slab by screws.
6.1.4 A suitable compressed air flow meter can measure the air flow that
produces a vibration frequency of 20 800 (347 Hz) times per minute.
6.1.5 Six stainless steel test vessels.
6.1.6 A stainless steel plate with 6 holes that is suitable for the installation of
the test vessel, which is fixed on the slab by a fixture.
6.1.7 Stopwatch.
1 Such as K13 that is produced by ERKALAITEOY in Helsinki, Finland. This information is given for the
convenience of users of this part, rather than endorsement of the product.
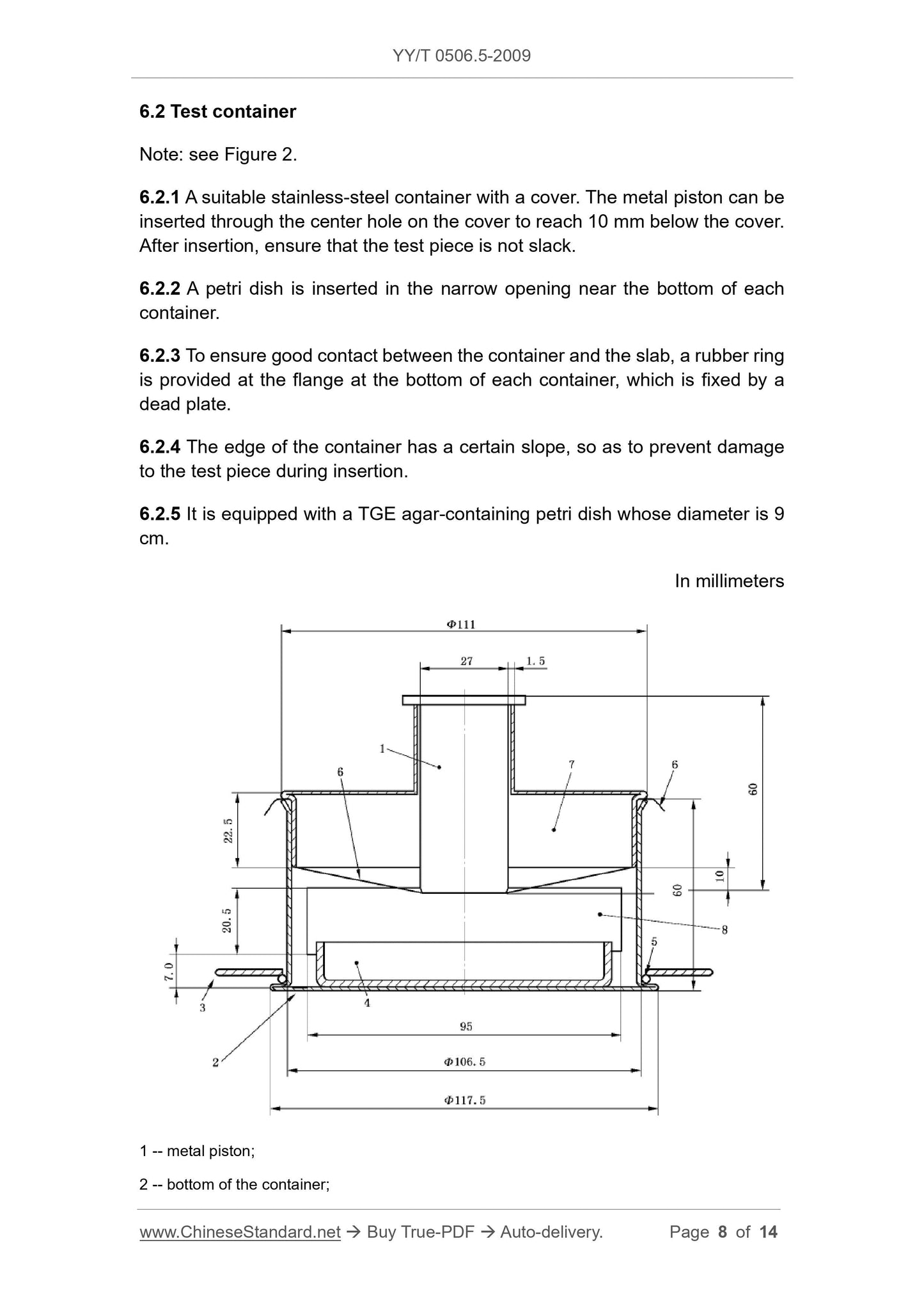
3 -- dead plate;
4 -- petri dish.
5 -- rubber ring;
6 -- test piece;
7 -- the cover;
8 -- inserting narrow opening of the petri dish.
Figure 2 -- Test container
6.3 How to add bacterial spores to talc powder
6.3.1 Materials
6.3.1.1 50 g ± 0.5 g of talc powder (95% < 15 μm)2.
6.3.1.2 Ethanol contains pure bacillus subtilis ATCC 9372 spores 3 whose
concentration is ≥ 109/mL.
6.3.1.3 TGE agar dish.
6.3.2 Procedure
6.3.2.1 Place 50 g of talc powder in a suitable container; dry heat at 160 °C for
2+1 0 h.
6.3.2.2 Open an ampoule of 5 mL alcohol spore solution.
6.3.2.3 Add the spore solution to the talc powder 50 times (50×100 μL)
6.3.2.4 Use a vortex vibrator to vibrate the closed container every time when it
is added.
6.3.2.5 Place the container open in a desiccator that contains silicone gel and
dry at room temperature for 2 d ~ 3 d.
6.3.2.6 Weigh the container before and after drying to ensure complete drying.
6.3.2.7 Culture the spore talc powder mixture on TGE agar at 35°C; evaluate
its bioburden (perform three tests in total, repeat twice each time); express as
the number of spores in talc powder CFU/g.
2 GB 15342 stipulates the requirements of talc powder, and the specification suitable for this test is 800
mesh.
3 For example, SIMICON GmbH, Schumacherring 12, D-21736 Munchen, fax +498967336622. E-mail:
info@simicon.de. This information is given for the convenience of users of this part, rather than
endorsement of the product.
6.3.2.8 The final concentration should be 108 CFU/g talc powder; ensure that
the spores are evenly distributed in the talc powder.
7 Procedure
7.1 Cut 12 200 mm × 200 mm test pieces.
7.2 Put the test pieces into sterilization bags and sterilize according to the
method that is given by the manufacturer.
7.3 Place each container in a sterilization bag and sterilize.
7.4 Use dead plates and fixtures to fix the bottom of the container to the slab
reliably.
7.5 Remove the test piece from the bag by aseptic technique and place it on
the mouth of each test container.
7.6 Fix the cover on the container by pushing the piston down, so that the test
piece is fixed under a controlled degree of relaxation.
7.7 Remove the piston.
7.8 Add 0.5 g + 0.1 g of contaminated talc powder to the test piece through the
piston mouth; add the uncontaminated talc powder to the sixth container as a
control.
7.9 Use a film to seal the piston mouth.
7.10 Cover a small plastic bag to each container.
7.11 Insert uncovered petri dishes through the narrow openings at the bottom
of each container.
7.12 Use adhesive tape to close the narrow opening.
7.13 Vibrate for 30 minutes at a vibration frequency of 20 800 times per minute.
7.14 Remove the plastic bag and the adhesive tape.
7.15 Insert the cover of the petri dish through the narrow opening.
7.16 Take out the petri dish and incubate at 35 °C for 24 h.
7.17 Count the formed bacterial colonies. The read of the control dish should
be 0. Otherwise, it indicates that there is foreign contamination, and the test
should be terminated.
Get QUOTATION in 1-minute: Click YY/T 0506.5-2009
Historical versions: YY/T 0506.5-2009
Preview True-PDF (Reload/Scroll if blank)
YY/T 0506.5-2009: Surgical drapes, gowns and clean air suits for patients, clinical staff and equipment. Part 5: Test method for resistance to dry microbial penetration
YY/T 0506.5-2009
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.140
C 46
Surgical drapes, gowns and clean air suits for
patients, clinical staff and equipment - Part 5: Test
method for resistance to dry microbial penetration
(ISO 22612:2005, MOD)
ISSUED ON: JUNE 16, 2009
IMPLEMENTED ON: DECEMBER 01, 2010
Issued by: China Food and Drug Administration
Table of Contents
Foreword ... 3
Introduction ... 4
1 Scope ... 5
2 Normative references ... 5
3 Terms and definitions ... 5
4 Principle ... 5
5 Test conditions ... 6
6 Equipment ... 6
7 Procedure ... 10
8 Test report ... 11
Appendix A (Informative) TGE agar medium ... 12
Appendix NA (Informative) Technical differences between this Standard and
ISO 22612:2005 and their reasons ... 13
References ... 14
Surgical drapes, gowns and clean air suits for
patients, clinical staff and equipment - Part 5: Test
method for resistance to dry microbial penetration
1 Scope
This part of YY/T 0506 stipulates the test method that is used to evaluate the
resistance of permeability to bacterial particles of barrier materials.
2 Normative references
The terms in the following documents become the terms of this part by
reference to this part of YY/T 0506. For dated references, all subsequent
amendments (not including errata content) or revisions do not apply to this part.
However, parties to agreements that are based on this part are encouraged to
study whether the latest versions of these documents can be used. For undated
references, the latest edition applies to this part.
YY/T 0506.1, Surgical drapes, gowns and clean air suits for patients, clinical
staff and equipment - Part 1: General requirements for manufacturers,
processors and products
3 Terms and definitions
The terms and definitions of YY/T 0506.1 apply to this part of YY/T 0506.
4 Principle
This test is carried out on test pieces that are respectively fixed on a container.
Among these containers, 5 containers that carry bacillus subtilis talc powder
and 1 container that is added with untainted talc powder are used as controls.
Insert a petri dish at the bottom of each container that is close to the bottom of
the test piece.
The equipment that supports the container is oscillated by a gas ball oscillator;
all the talc powders that penetrate the test piece fall onto the petri dish; take out
the petri dish and cultivate.
Count the growing bacterial colonies.
5 Test conditions
Adjust the state and test the samples at 20 °C ± 2 °C and the relative humidity
of 65% ± 5%.
6 Equipment
6.1 General structure
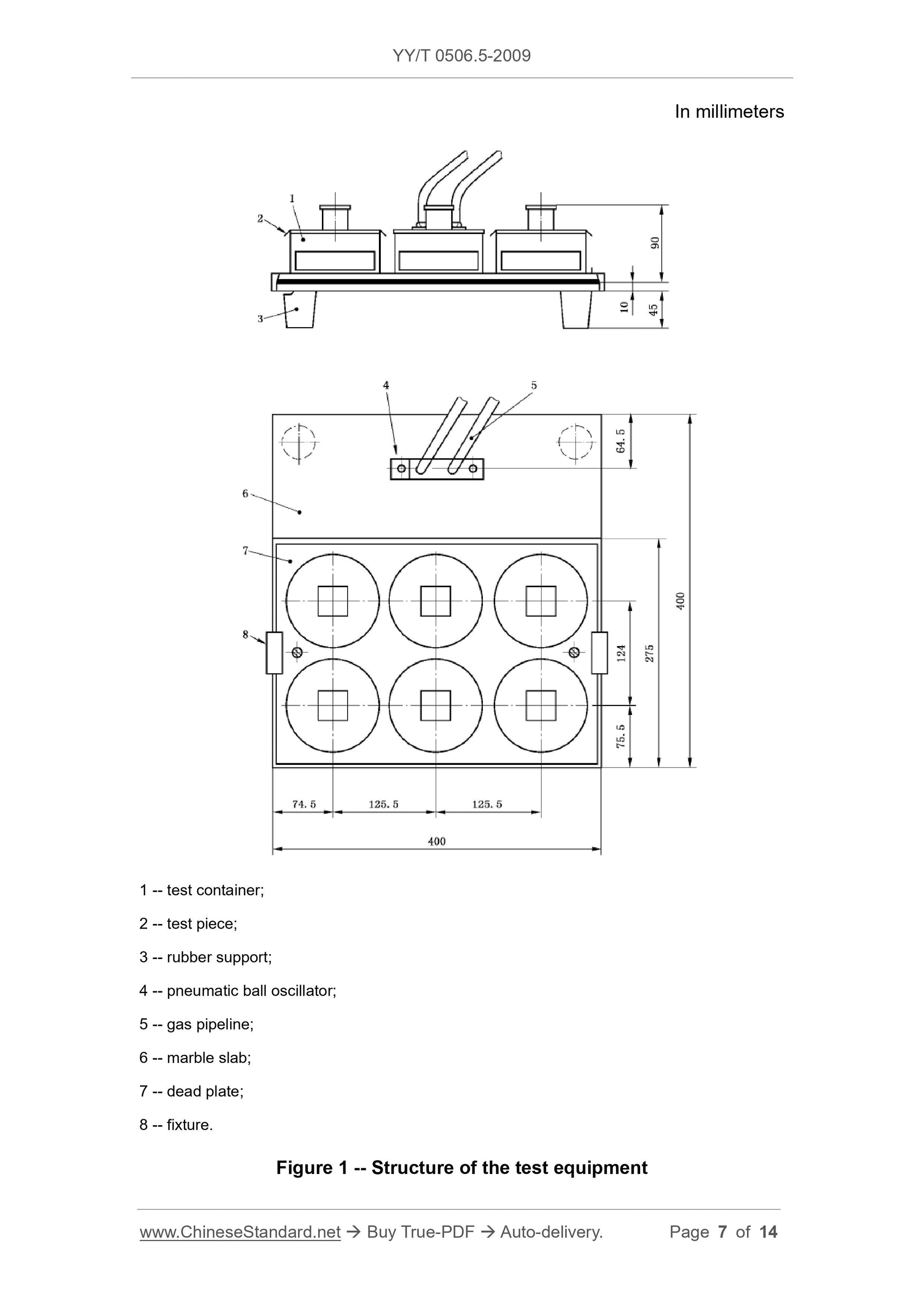
Note: see Figure 1.
6.1.1 10 mm thick stone slab, such as marble, 40 cm × 40 cm, with 4 rubber
supports at each corner.
6.1.2 Pneumatic ball oscillator1, that can produce 20800 vibrations per minute;
the force is 650 N.
6.1.3 An oscillator that is connected to one side of the upper surface of the
marble slab by screws.
6.1.4 A suitable compressed air flow meter can measure the air flow that
produces a vibration frequency of 20 800 (347 Hz) times per minute.
6.1.5 Six stainless steel test vessels.
6.1.6 A stainless steel plate with 6 holes that is suitable for the installation of
the test vessel, which is fixed on the slab by a fixture.
6.1.7 Stopwatch.
1 Such as K13 that is produced by ERKALAITEOY in Helsinki, Finland. This information is given for the
convenience of users of this part, rather than endorsement of the product.
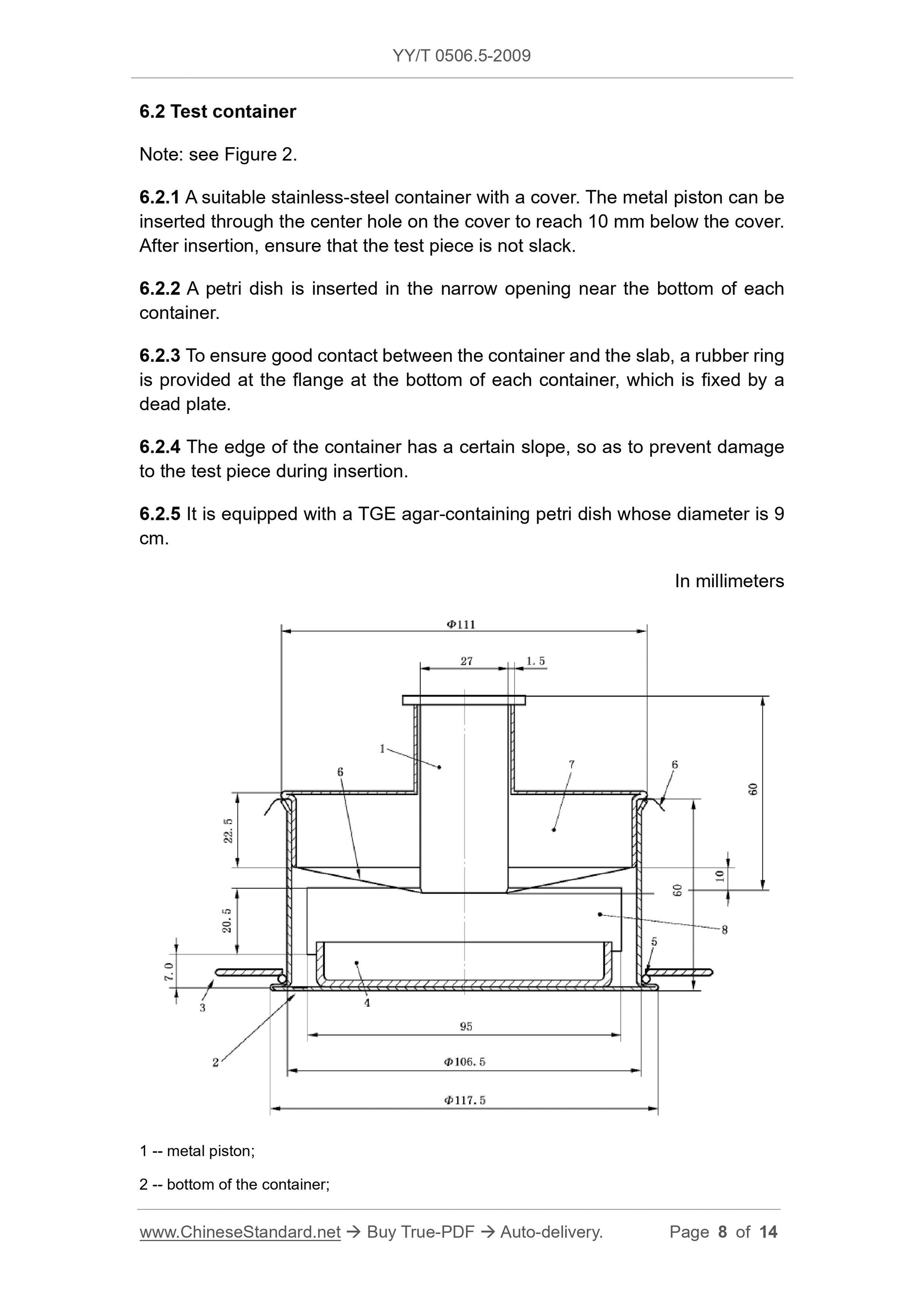
3 -- dead plate;
4 -- petri dish.
5 -- rubber ring;
6 -- test piece;
7 -- the cover;
8 -- inserting narrow opening of the petri dish.
Figure 2 -- Test container
6.3 How to add bacterial spores to talc powder
6.3.1 Materials
6.3.1.1 50 g ± 0.5 g of talc powder (95% < 15 μm)2.
6.3.1.2 Ethanol contains pure bacillus subtilis ATCC 9372 spores 3 whose
concentration is ≥ 109/mL.
6.3.1.3 TGE agar dish.
6.3.2 Procedure
6.3.2.1 Place 50 g of talc powder in a suitable container; dry heat at 160 °C for
2+1 0 h.
6.3.2.2 Open an ampoule of 5 mL alcohol spore solution.
6.3.2.3 Add the spore solution to the talc powder 50 times (50×100 μL)
6.3.2.4 Use a vortex vibrator to vibrate the closed container every time when it
is added.
6.3.2.5 Place the container open in a desiccator that contains silicone gel and
dry at room temperature for 2 d ~ 3 d.
6.3.2.6 Weigh the container before and after drying to ensure complete drying.
6.3.2.7 Culture the spore talc powder mixture on TGE agar at 35°C; evaluate
its bioburden (perform three tests in total, repeat twice each time); express as
the number of spores in talc powder CFU/g.
2 GB 15342 stipulates the requirements of talc powder, and the specification suitable for this test is 800
mesh.
3 For example, SIMICON GmbH, Schumacherring 12, D-21736 Munchen, fax +498967336622. E-mail:
info@simicon.de. This information is given for the convenience of users of this part, rather than
endorsement of the product.
6.3.2.8 The final concentration should be 108 CFU/g talc powder; ensure that
the spores are evenly distributed in the talc powder.
7 Procedure
7.1 Cut 12 200 mm × 200 mm test pieces.
7.2 Put the test pieces into sterilization bags and sterilize according to the
method that is given by the manufacturer.
7.3 Place each container in a sterilization bag and sterilize.
7.4 Use dead plates and fixtures to fix the bottom of the container to the slab
reliably.
7.5 Remove the test piece from the bag by aseptic technique and place it on
the mouth of each test container.
7.6 Fix the cover on the container by pushing the piston down, so that the test
piece is fixed under a controlled degree of relaxation.
7.7 Remove the piston.
7.8 Add 0.5 g + 0.1 g of contaminated talc powder to the test piece through the
piston mouth; add the uncontaminated talc powder to the sixth container as a
control.
7.9 Use a film to seal the piston mouth.
7.10 Cover a small plastic bag to each container.
7.11 Insert uncovered petri dishes through the narrow openings at the bottom
of each container.
7.12 Use adhesive tape to close the narrow opening.
7.13 Vibrate for 30 minutes at a vibration frequency of 20 800 times per minute.
7.14 Remove the plastic bag and the adhesive tape.
7.15 Insert the cover of the petri dish through the narrow opening.
7.16 Take out the petri dish and incubate at 35 °C for 24 h.
7.17 Count the formed bacterial colonies. The read of the control dish should
be 0. Otherwise, it indicates that there is foreign contamination, and the test
should be terminated.
Share