1
/
von
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 0987.5-2016 English PDF (YYT0987.5-2016)
YY/T 0987.5-2016 English PDF (YYT0987.5-2016)
Normaler Preis
$140.00 USD
Normaler Preis
Verkaufspreis
$140.00 USD
Grundpreis
/
pro
Versand wird beim Checkout berechnet
Verfügbarkeit für Abholungen konnte nicht geladen werden
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY/T 0987.5-2016
Historical versions: YY/T 0987.5-2016
Preview True-PDF (Reload/Scroll if blank)
YY/T 0987.5-2016: Implants for surgery--Magnetic resonance compatibility--Part 5: Magnetically induced torque test method
YY/T 0987.5-2016
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.40
C 35
Implants for Surgery - Magnetic Resonance
Compatibility - Part 5. Magnetically Induced
Torque Test Method
外科植入物 磁共振兼容性
ISSUED ON. MARCH 23, 2016
IMPLEMENTED ON. JANUARY 1, 2017
Issued by. China Food and Drug Administration
Table of Contents
Foreword ... 3
1 Scope ... 5
2 Normative References ... 5
3 Terms and Definitions ... 6
4 Overview of Test Method ... 8
5 Significance and Application ... 9
6 Instruments and Equipment ... 9
7 Test Sample ... 11
8 Procedures ... 11
9 Calculation ... 12
10 Report ... 12
Appendix A (Informative) Fundamental Principle ... 14
Bibliography ... 19
Implants for Surgery - Magnetic Resonance
Compatibility - Part 5. Magnetically Induced
Torque Test Method
1 Scope
This Part of YY/T 0987 includes test method for magnetically induced torque generated
by medical devices as a result of static magnetic field in magnetic resonance
environment; a comparison of magnetically induced torque and medical devices’
gravity moment.
This Part does not involve other possible safety questions. These safety questions
include, but are not limited to, magnetically induced displacement force generated by
the spatial gradient of magnetic field, radio frequency heating and radio frequency
induced heating, noise, interaction among medical devices, functions of medical
devices and magnetic resonance system.
In this Part, torque refers to static magnetic torque generated by MRI static magnetic
field and implant’s magnetic moment. This Part does not include dynamic torque
generated by the rotation of medical device as a result of the interaction between the
static magnetic field and eddy current. Current in the wires might also generate torque.
The sensitivity of torque measurement device shall be more than 1/10 of gravity
moment; gravity moment equals the arithmetic product of the maximum linear size and
the weight of medical device.
This Part does not attempt to elaborate all the involved safety questions, even though
those safety questions are related with the usage. Determining appropriate safety and
health specifications and clarifying the applicability of management limit before
application is the responsibility on the users of this Standard.
2 Normative References
The following documents are indispensable to the application of this Standard. In terms
of references with a specified date, only versions with a specified date are applicable
to this Standard. The latest version (including all the modifications) of references
without a specified date is also applicable to this Standard.
YY/T 0987.1 Implants for Surgery - Magnetic Resonance Compatibility - Part 1.
Safety Marking
YY/T 0987.2 Implants for Surgery - Magnetic Resonance Compatibility - Part 2.
images and/or spectrograms, other physical parameters can also be obtained.
3.7 Magnetic Resonance (MR) Environment
Magnetic resonance environment refers to the space within 0.5 mT (5G) line in MR
system, including the whole three-dimensional space around MR scanner. When 0.5
mT line is included in Faraday cage, the whole space shall be deemed as magnetic
resonance (MR) environment.
3.8 Magnetic Resonance Equipment
MR Equipment
Magnetic resonance equipment refers to medical electrical equipment that is expected
to be applied to in vivo magnetic resonance examination. Magnetic resonance
equipment includes all hardware and software parts from main power to display
monitor. Magnetic resonance equipment is programmable electrical medical system
(PEMS).
3.9 Magnetic Resonance Examination
MR Examination
Magnetic resonance examination refers to the process of gathering patients’ data
through magnetic resonance.
3.10 Magnetic Resonance Imaging; MRI
Magnetic resonance imaging refers to the imaging technology, which utilizes static
time-varying magnetic field to generate resonance of atomic nucleus to obtain tissue
images.
3.11 Magnetic Resonance System
MR System
Magnetic resonance system refers to the combination of magnetic resonance
equipment, accessories (including display, control and energy supply devices) and
controlled entry zone (if provided).
3.12 Magnetically Induced Displacement Force
Magnetically induced displacement force refers to the force on magnetic object in
spatial gradient magnetic field. This force will lead to movement of magnetic object in
the gradient field.
3.13 Magnetically Induced Torque
Magnetically induced torque refers to the torque generated by magnetic object in
in the middle of the magnetic object of magnetic resonance equipment, where
magnetic field is even. The size of torque depends on the loading tray’s deflection
angle relative to an equilibrium position. By rotating the supporting torsional spring and
the frame of the loading tray, the deflection angle of implant can be measured. Thus,
the torque can be calculated. Compare the maximum torque with the severest gravity
moment of medical device; the severest gravity moment equals to the arithmetic
product of medical device’s maximum linear size and weight.
5 Significance and Application
The test method in this Part is is one of the test methods of determining whether the
existence of medical devices would lead to patients’ injury in MR examination or MR
environment. Other safety questions that need to be described include, but are not
limited to, magnetically induced displacement force (refer to YY/T 0987.2) and radio
frequency induced heating (refer to YY/T 0987.4). In order to guarantee the safety of
medical devices in magnetic resonance environment, terms and markings stipulated
in YY/T 0987.1 shall be applied to the marking of medical devices.
If the maximum torque is less than the arithmetic product of medical device’s maximum
size and weight, then, magnetically induced torque is less than the severest gravity
moment generated by gravity. Under this circumstance, it can be deemed that the risk
of magnetically induced torque is not higher than risks of daily activities in the earth’s
gravity field. This is merely conservative estimation; it is possible that a larger torque
also would not bring any hazard to patients.
This test is insufficient to prove medical devices’ safety in magnetic resonance
environment.
The sensitivity of torque measurement device shall be more than 1/10 of gravity
moment; gravity moment equals the arithmetic product of the maximum linear size and
the weight of medical device.
In this Part, torque refers to static magnetic torque generated by MRI static magnetic
field and implant’s magnetic moment. This Part does not include dynamic torque
generated by the rotation of medical device as a result of the interaction between the
static magnetic field and eddy current. Current in the wires might also generate torque.
6 Instruments and Equipment
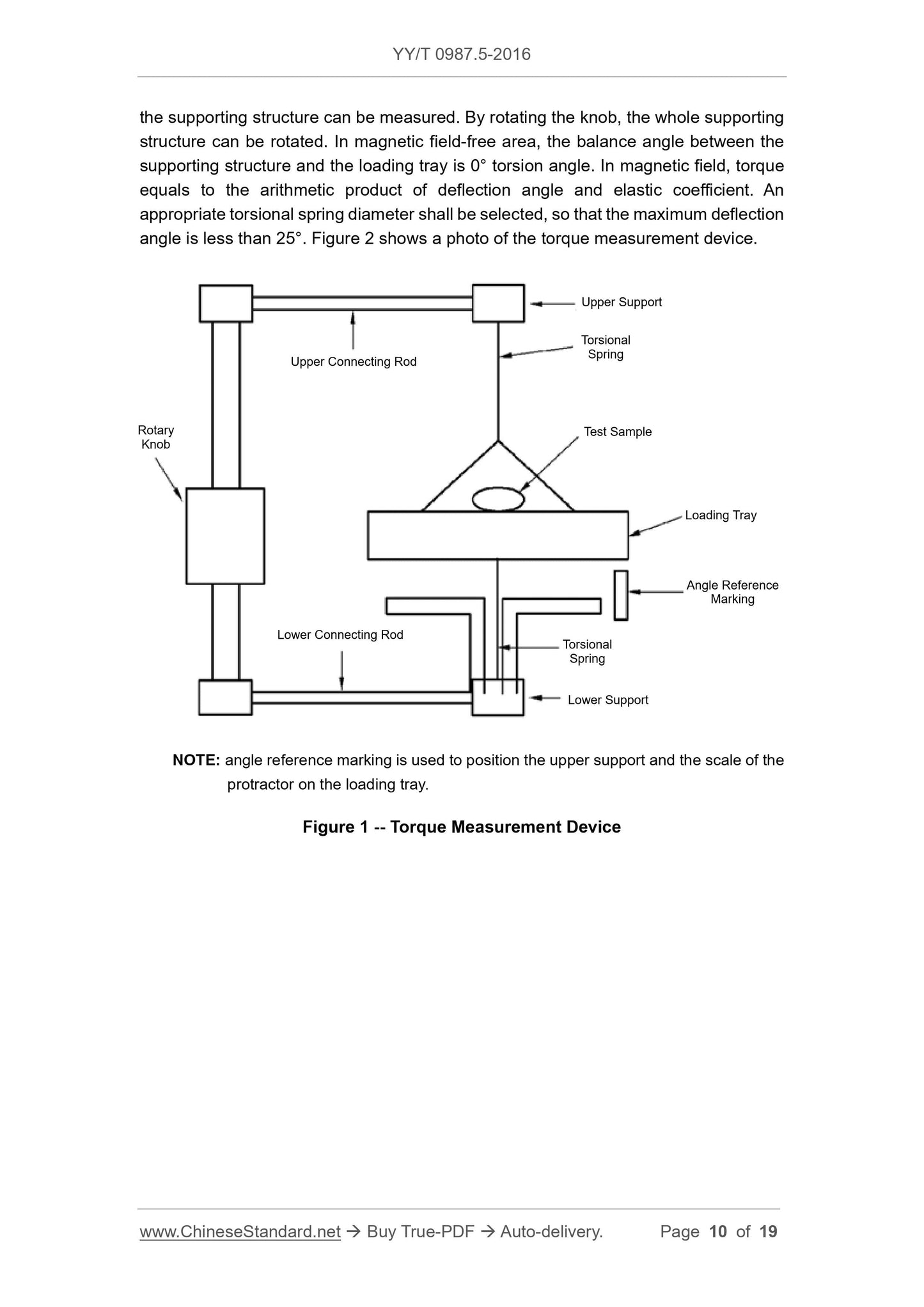
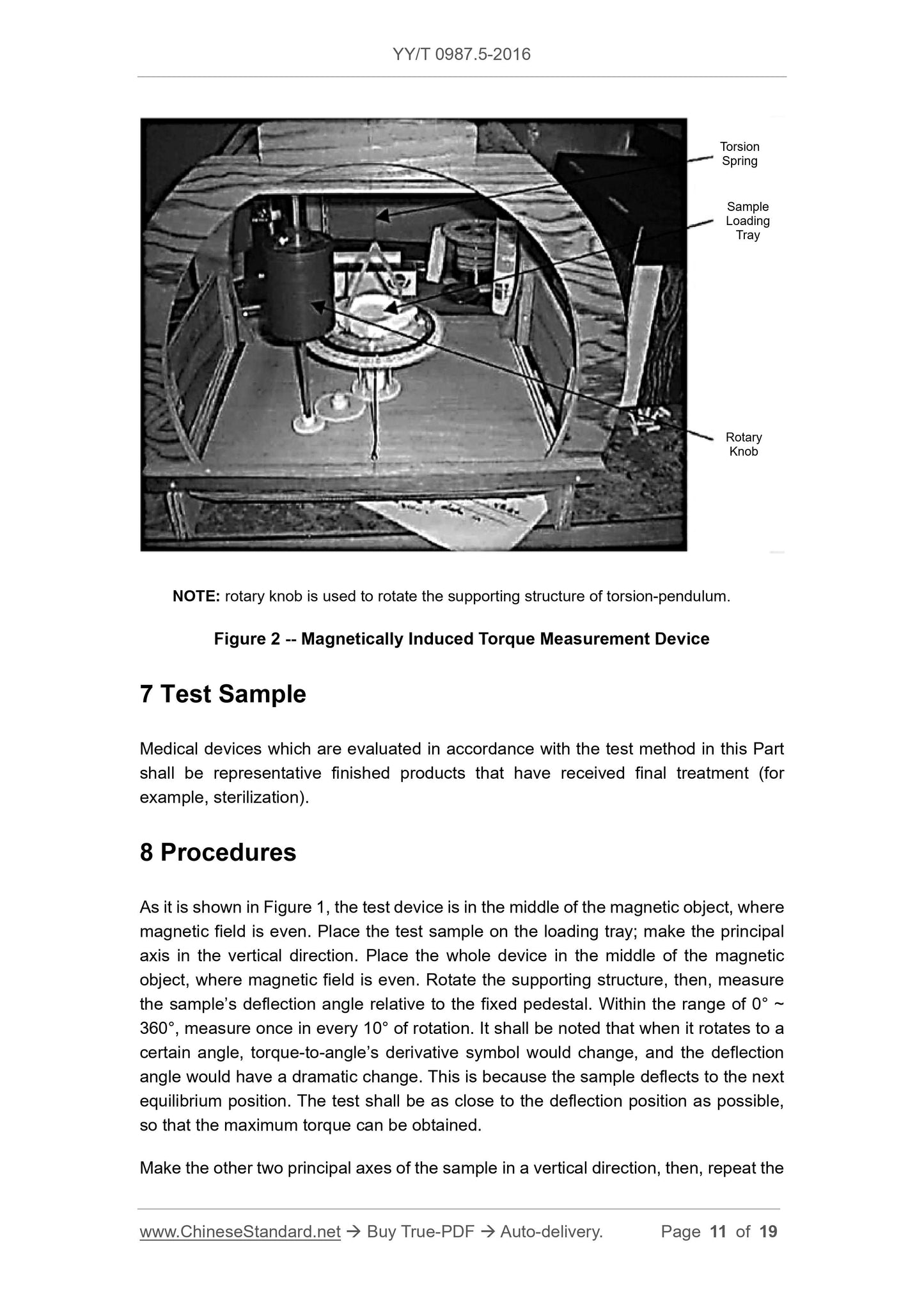
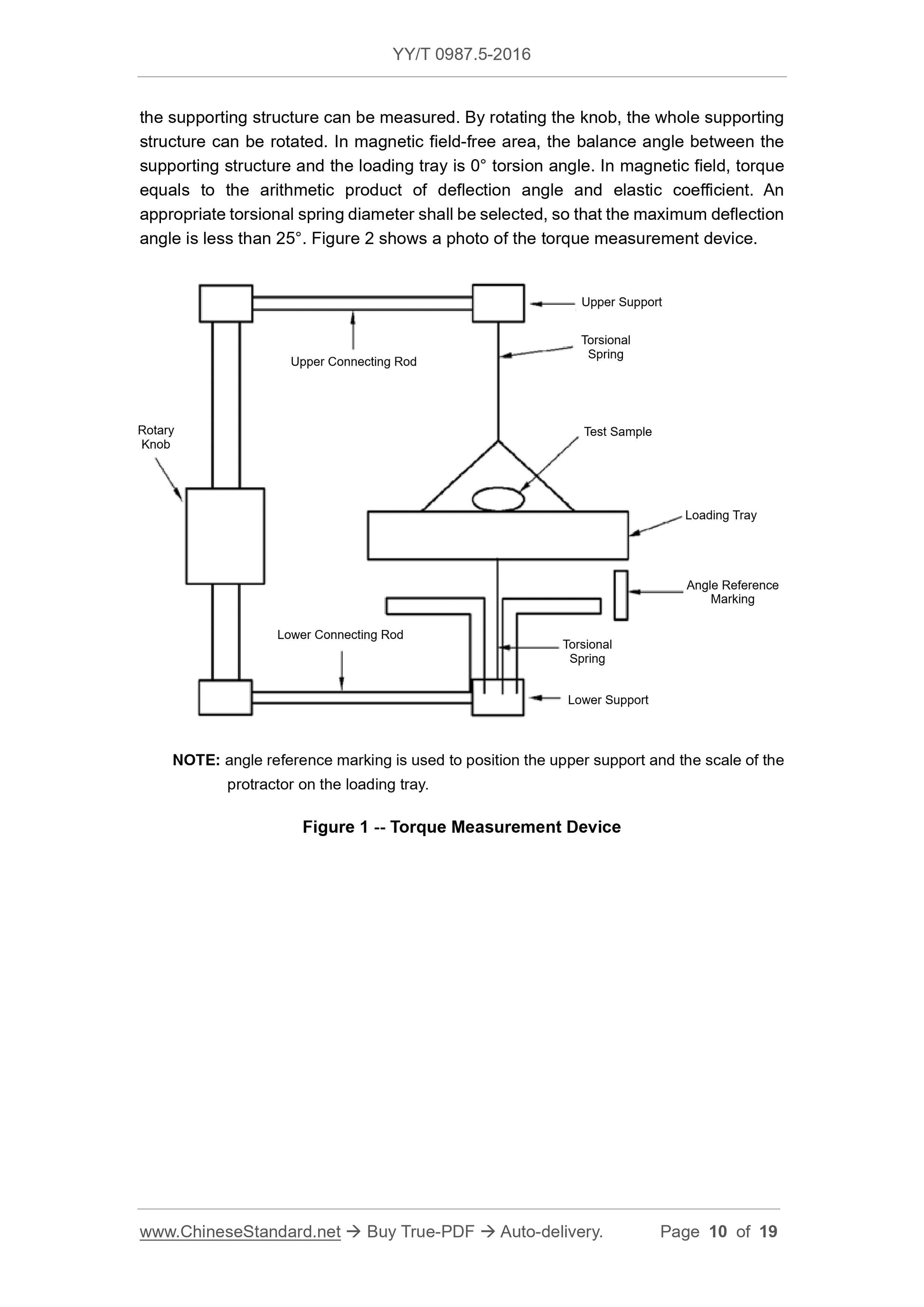
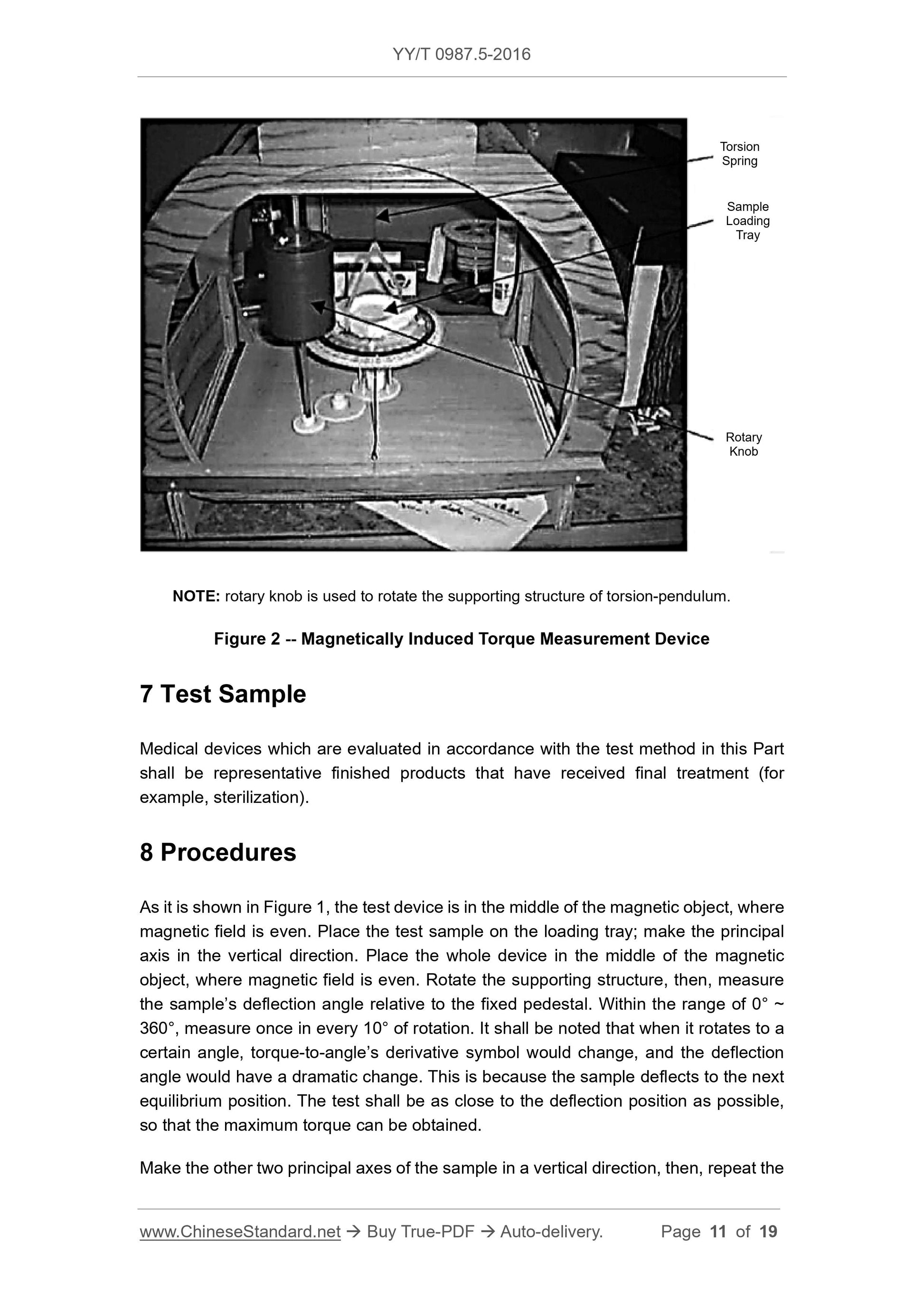
Test device is shown in Figure 1. The device is constituted of a solid structure, including
a loading tray supported by torsional spring. The device is completely made of non-
ferromagnetic materials. Test sample shall be pasted or bundled on the loading tray. A
protractor (division value. 1°) shall be fixated on a supporting structure. There is a
marking on the loading tray, through which, the included angle of the loading tray and
above test process.
In terms of conductors, try to place them in accordance with the ...
Get QUOTATION in 1-minute: Click YY/T 0987.5-2016
Historical versions: YY/T 0987.5-2016
Preview True-PDF (Reload/Scroll if blank)
YY/T 0987.5-2016: Implants for surgery--Magnetic resonance compatibility--Part 5: Magnetically induced torque test method
YY/T 0987.5-2016
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.40
C 35
Implants for Surgery - Magnetic Resonance
Compatibility - Part 5. Magnetically Induced
Torque Test Method
外科植入物 磁共振兼容性
ISSUED ON. MARCH 23, 2016
IMPLEMENTED ON. JANUARY 1, 2017
Issued by. China Food and Drug Administration
Table of Contents
Foreword ... 3
1 Scope ... 5
2 Normative References ... 5
3 Terms and Definitions ... 6
4 Overview of Test Method ... 8
5 Significance and Application ... 9
6 Instruments and Equipment ... 9
7 Test Sample ... 11
8 Procedures ... 11
9 Calculation ... 12
10 Report ... 12
Appendix A (Informative) Fundamental Principle ... 14
Bibliography ... 19
Implants for Surgery - Magnetic Resonance
Compatibility - Part 5. Magnetically Induced
Torque Test Method
1 Scope
This Part of YY/T 0987 includes test method for magnetically induced torque generated
by medical devices as a result of static magnetic field in magnetic resonance
environment; a comparison of magnetically induced torque and medical devices’
gravity moment.
This Part does not involve other possible safety questions. These safety questions
include, but are not limited to, magnetically induced displacement force generated by
the spatial gradient of magnetic field, radio frequency heating and radio frequency
induced heating, noise, interaction among medical devices, functions of medical
devices and magnetic resonance system.
In this Part, torque refers to static magnetic torque generated by MRI static magnetic
field and implant’s magnetic moment. This Part does not include dynamic torque
generated by the rotation of medical device as a result of the interaction between the
static magnetic field and eddy current. Current in the wires might also generate torque.
The sensitivity of torque measurement device shall be more than 1/10 of gravity
moment; gravity moment equals the arithmetic product of the maximum linear size and
the weight of medical device.
This Part does not attempt to elaborate all the involved safety questions, even though
those safety questions are related with the usage. Determining appropriate safety and
health specifications and clarifying the applicability of management limit before
application is the responsibility on the users of this Standard.
2 Normative References
The following documents are indispensable to the application of this Standard. In terms
of references with a specified date, only versions with a specified date are applicable
to this Standard. The latest version (including all the modifications) of references
without a specified date is also applicable to this Standard.
YY/T 0987.1 Implants for Surgery - Magnetic Resonance Compatibility - Part 1.
Safety Marking
YY/T 0987.2 Implants for Surgery - Magnetic Resonance Compatibility - Part 2.
images and/or spectrograms, other physical parameters can also be obtained.
3.7 Magnetic Resonance (MR) Environment
Magnetic resonance environment refers to the space within 0.5 mT (5G) line in MR
system, including the whole three-dimensional space around MR scanner. When 0.5
mT line is included in Faraday cage, the whole space shall be deemed as magnetic
resonance (MR) environment.
3.8 Magnetic Resonance Equipment
MR Equipment
Magnetic resonance equipment refers to medical electrical equipment that is expected
to be applied to in vivo magnetic resonance examination. Magnetic resonance
equipment includes all hardware and software parts from main power to display
monitor. Magnetic resonance equipment is programmable electrical medical system
(PEMS).
3.9 Magnetic Resonance Examination
MR Examination
Magnetic resonance examination refers to the process of gathering patients’ data
through magnetic resonance.
3.10 Magnetic Resonance Imaging; MRI
Magnetic resonance imaging refers to the imaging technology, which utilizes static
time-varying magnetic field to generate resonance of atomic nucleus to obtain tissue
images.
3.11 Magnetic Resonance System
MR System
Magnetic resonance system refers to the combination of magnetic resonance
equipment, accessories (including display, control and energy supply devices) and
controlled entry zone (if provided).
3.12 Magnetically Induced Displacement Force
Magnetically induced displacement force refers to the force on magnetic object in
spatial gradient magnetic field. This force will lead to movement of magnetic object in
the gradient field.
3.13 Magnetically Induced Torque
Magnetically induced torque refers to the torque generated by magnetic object in
in the middle of the magnetic object of magnetic resonance equipment, where
magnetic field is even. The size of torque depends on the loading tray’s deflection
angle relative to an equilibrium position. By rotating the supporting torsional spring and
the frame of the loading tray, the deflection angle of implant can be measured. Thus,
the torque can be calculated. Compare the maximum torque with the severest gravity
moment of medical device; the severest gravity moment equals to the arithmetic
product of medical device’s maximum linear size and weight.
5 Significance and Application
The test method in this Part is is one of the test methods of determining whether the
existence of medical devices would lead to patients’ injury in MR examination or MR
environment. Other safety questions that need to be described include, but are not
limited to, magnetically induced displacement force (refer to YY/T 0987.2) and radio
frequency induced heating (refer to YY/T 0987.4). In order to guarantee the safety of
medical devices in magnetic resonance environment, terms and markings stipulated
in YY/T 0987.1 shall be applied to the marking of medical devices.
If the maximum torque is less than the arithmetic product of medical device’s maximum
size and weight, then, magnetically induced torque is less than the severest gravity
moment generated by gravity. Under this circumstance, it can be deemed that the risk
of magnetically induced torque is not higher than risks of daily activities in the earth’s
gravity field. This is merely conservative estimation; it is possible that a larger torque
also would not bring any hazard to patients.
This test is insufficient to prove medical devices’ safety in magnetic resonance
environment.
The sensitivity of torque measurement device shall be more than 1/10 of gravity
moment; gravity moment equals the arithmetic product of the maximum linear size and
the weight of medical device.
In this Part, torque refers to static magnetic torque generated by MRI static magnetic
field and implant’s magnetic moment. This Part does not include dynamic torque
generated by the rotation of medical device as a result of the interaction between the
static magnetic field and eddy current. Current in the wires might also generate torque.
6 Instruments and Equipment
Test device is shown in Figure 1. The device is constituted of a solid structure, including
a loading tray supported by torsional spring. The device is completely made of non-
ferromagnetic materials. Test sample shall be pasted or bundled on the loading tray. A
protractor (division value. 1°) shall be fixated on a supporting structure. There is a
marking on the loading tray, through which, the included angle of the loading tray and
above test process.
In terms of conductors, try to place them in accordance with the ...
Share