PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY 1075-2007 English PDF (YY1075-2007)
YY 1075-2007 English PDF (YY1075-2007)
Precio habitual
$150.00 USD
Precio habitual
Precio de oferta
$150.00 USD
Precio unitario
/
por
Los gastos de envío se calculan en la pantalla de pago.
No se pudo cargar la disponibilidad de retiro
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY 1075-2007
Historical versions: YY 1075-2007
Preview True-PDF (Reload/Scroll if blank)
YY 1075-2007: Rigid hysteroscope
YY 1075-2007
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.70
C 40
Replacing YY 91075-1999
Rigid hysteroscope
硬性宫腔内窥镜
ISSUED ON: JULY 02, 2007
IMPLEMENTED ON: MARCH 01, 2008
Issued by: China Food and Drug Administration
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative references ... 4
3 Classification and composition ... 5
4 Requirements ... 5
5 Test methods ... 8
6 Inspection rules ... 17
7 Signs, labels and instruction manual ... 18
8 Packaging, transportation, storage ... 20
Appendix A (Normative) Safety requirements for interconnection with medical
electrical equipment ... 21
Foreword
This standard is a revision of YY 91075-1999 “Hysteroscope”.
As compared with YY 91075-1999, the main changes of this standard are as
follows:
- ADD the classification and marking;
- STANDARDIZE the standard’s name;
- DIVIDE the hysteroscope into two types: inspection hysteroscope and
surgery hysteroscope. Surgery hysteroscope is further divided into two
types: integrated and split;
- MAKE specific parameters and technical requirements for the two types of
hysteroscope, respectively;
- ADD the biocompatibility requirements.
The electrical connection part fully implements the relevant provisions of GB
9706.1-1995 “Medical electrical equipment - Part 1: General requirements for
safety” and GB 9706.19-2000 “Medical electrical equipment - Part 2: Particular
requirements for the safety of endoscopic equipment”. The specific content is
given in the form of Appendix A (normative).
Appendix A of this standard is a normative appendix.
This standard was approved by the China Food and Drug Administration.
This standard was proposed by and shall be under the jurisdiction of the
National Technical Committee for Standardization of Medical Optics and
Instruments.
Drafting organization of this standard: Shenyang Shenda Endoscope Co., Ltd.
The main drafters of this standard: Jiang Kerang, Gao Mingxian, Zhang
Chang’an.
This standard replaces the standard previously issued as follows:
- ZB C36001-1985;
- YY 91075-1999.
Rigid hysteroscope
1 Scope
This standard specifies the classification and marking, requirements, test
methods, inspection rules, signs, labels and instructions for use, packaging,
transportation, storage of rigid hysteroscope.
This standard applies to rigid hysteroscope (hereinafter referred to as
hysteroscope). Hysteroscope is mainly used in the medical clinical diagnosis of
uterine cavity disease and treatment together with surgery instruments.
This standard does not apply to high-frequency electric hysteroscope.
2 Normative references
The provisions in following documents become the provisions of this standard
through reference in this standard. For the dated references, the subsequent
amendments (excluding corrections) or revisions do not apply to this standard;
however, parties who reach an agreement based on this standard are
encouraged to study if the latest versions of these documents are applicable.
For undated references, the latest edition of the referenced document applies.
GB/T 191-2000 Packaging - Pictorial marking for handling of goods
GB/T 2829-2002 Sampling procedures and tables for periodic inspection by
attributes (Apply to inspection of process stability)
GB/T 6463-2005 Metallic and other inorganic coatings - Review of methods
of measurement of thickness
GB 9706.1-1995 Medical electrical equipment - Part 1: General
requirements for safety (idt IEC 601-1:1988)
GB 9706.19-2000 Medical electrical equipment - Part 2: Particular
requirements for the safety of endoscopic equipment (idt IEC 60601-2-
18:1996)
GB 11244-2005 General requirements for the medical endoscope and
endoscope accessories
GB/T 14710-1993 The environmental requirements and test methods for
medical electrical equipment
GB/T 16886.1-2001 Biological evaluation of medical devices - Part 1:
Evaluation and testing (idt ISO 10993-1:1997)
GB/T 16886.5-2003 Biological evaluation of medical devices - Part 5: Test
for in vitro cytotoxicity (ISO 10993-5: 1999, IDT)
GB/T 16886.10-2005 Biological evaluation of medical devices - Part 10:
Tests for irritation and delayed-type hypersensitivity (ISO 10993-10:2002,
IDT)
YY 0068 General technical conditions for medical rigid endoscopes
YY 0076-1992 Coating classifications for metal product - Technical
conditions
YY 0466-2003 Medical devices - Symbols to be used with medical device
labels labelling and information to be supplied (ISO 15223:2000, IDT)
3 Classification and composition
3.1 Classification
Hysteroscope may be divided into two types: inspection hysteroscope and
surgery hysteroscope. Surgery hysteroscope is further divided into two types:
integrated and split.
3.2 Composition
3.2.1 The inspection hysteroscope consists of an endoscope, a sheath, an
obturator, a light guide.
3.2.2 The integrated surgery hysteroscope consists of an endoscope with an
instrument channel and a fluid-injection channel as well as a light guide; the
split surgery hysteroscope consists of an endoscope, a sheath, an obturator, a
manipulator, a light guide.
4 Requirements
4.1 Hysteroscope is a rigid endoscope product. In addition to the following
requirements, it shall also comply with the general requirements of YY 0068.
4.2 Surface and edge: The components of the hysteroscope shall be designed
so as not to cause any accidental injury to the human body, all surfaces must
be free of pores, cracks and burrs.
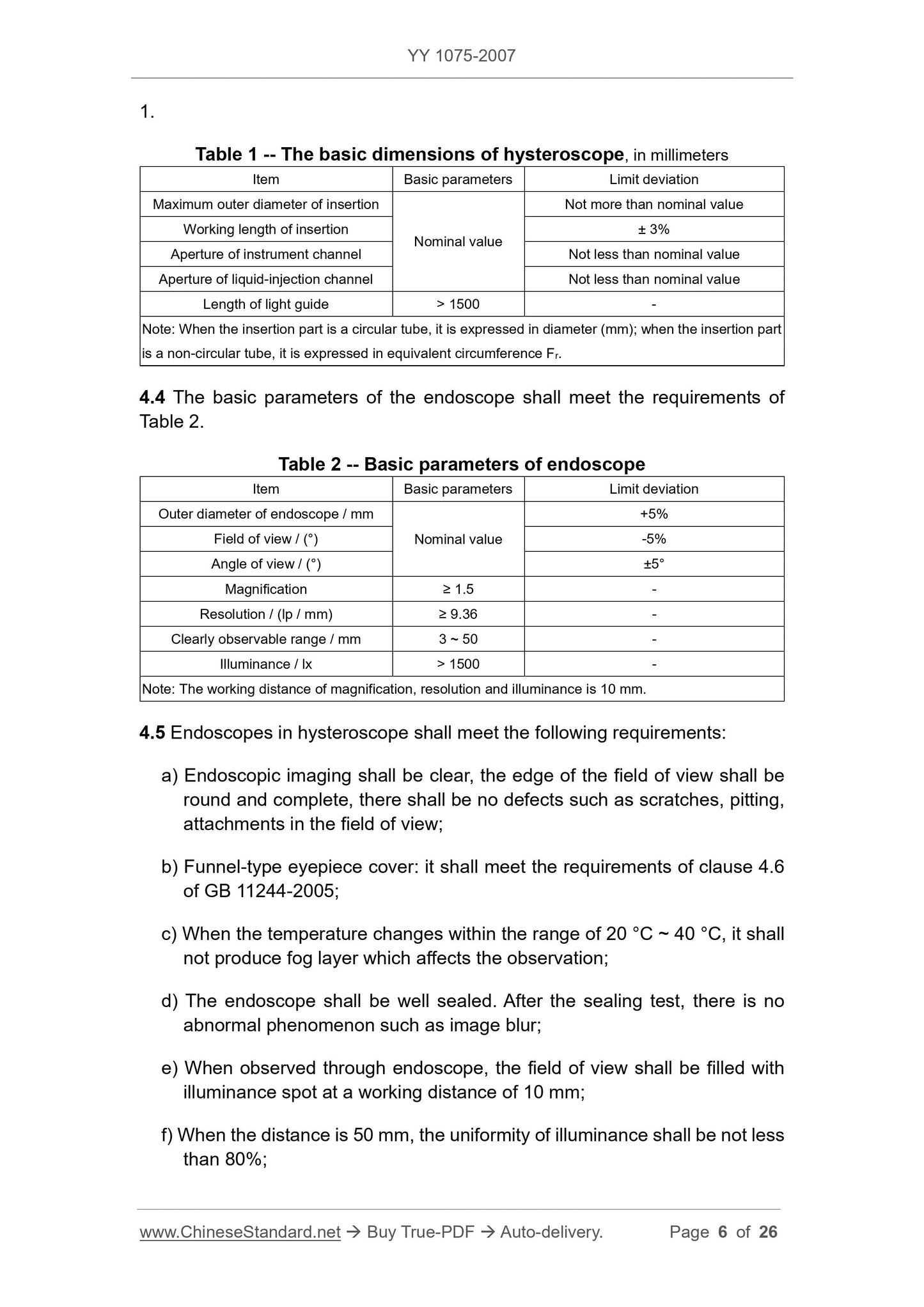
4.3 The basic dimensions of hysteroscope shall meet the requirements of Table
g) For the endoscope whose front end is curved, the curved end shall be firm
and reliable, the outer surface shall be smooth and tidy, there shall be no
light leakage;
h) For the endoscopes that can be sterilized by pressure steam, after the
test, the resolution and illuminance of the endoscope shall be not less than
95% of the original value.
4.6 The sheath and manipulator shall meet the following requirements:
a) The connection of the sheath, the manipulator, the obturator, the
endoscope shall be firm and reliable;
b) Each water-passing valve shall be flexible in rotation, the water-passing
valve and connection part shall be well sealed, the water seepage shall
be not more than five drops in 1 min;
c) The manipulator with the guide plate shall have flexible guiding plates, the
rotation of the rotating hand-wheel shall be flexible and reliable;
d) The endoscope or the instrument channel of the manipulator, the water-
injection channel shall be clean and unobstructed. The instrument channel
shall enable the matching surgery instruments to pass smoothly, the
instrument’s head shall be located in the field of view.
4.7 The flowrate of the hysteroscope’s water injection channel shall not be less
than 130 mL/min.
4.8 The surface of the hysteroscope’s limiting stopper shall be smooth, reliable
to position, easy to move.
4.9 The welding part of the hysteroscope shall be firm and reliable, flat and
smooth, without the phenomenon of de-soldering or surfacing.
4.10 The plating of hysteroscope’s plated components shall comply with the
category-V grade-2 requirements of YY 0076-1992.
4.11 Hysteroscope is a medical device that is in short-term contact with the
damaged surface. The outer surface material of the insertion part shall be made
of materials that have...
Get QUOTATION in 1-minute: Click YY 1075-2007
Historical versions: YY 1075-2007
Preview True-PDF (Reload/Scroll if blank)
YY 1075-2007: Rigid hysteroscope
YY 1075-2007
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.70
C 40
Replacing YY 91075-1999
Rigid hysteroscope
硬性宫腔内窥镜
ISSUED ON: JULY 02, 2007
IMPLEMENTED ON: MARCH 01, 2008
Issued by: China Food and Drug Administration
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative references ... 4
3 Classification and composition ... 5
4 Requirements ... 5
5 Test methods ... 8
6 Inspection rules ... 17
7 Signs, labels and instruction manual ... 18
8 Packaging, transportation, storage ... 20
Appendix A (Normative) Safety requirements for interconnection with medical
electrical equipment ... 21
Foreword
This standard is a revision of YY 91075-1999 “Hysteroscope”.
As compared with YY 91075-1999, the main changes of this standard are as
follows:
- ADD the classification and marking;
- STANDARDIZE the standard’s name;
- DIVIDE the hysteroscope into two types: inspection hysteroscope and
surgery hysteroscope. Surgery hysteroscope is further divided into two
types: integrated and split;
- MAKE specific parameters and technical requirements for the two types of
hysteroscope, respectively;
- ADD the biocompatibility requirements.
The electrical connection part fully implements the relevant provisions of GB
9706.1-1995 “Medical electrical equipment - Part 1: General requirements for
safety” and GB 9706.19-2000 “Medical electrical equipment - Part 2: Particular
requirements for the safety of endoscopic equipment”. The specific content is
given in the form of Appendix A (normative).
Appendix A of this standard is a normative appendix.
This standard was approved by the China Food and Drug Administration.
This standard was proposed by and shall be under the jurisdiction of the
National Technical Committee for Standardization of Medical Optics and
Instruments.
Drafting organization of this standard: Shenyang Shenda Endoscope Co., Ltd.
The main drafters of this standard: Jiang Kerang, Gao Mingxian, Zhang
Chang’an.
This standard replaces the standard previously issued as follows:
- ZB C36001-1985;
- YY 91075-1999.
Rigid hysteroscope
1 Scope
This standard specifies the classification and marking, requirements, test
methods, inspection rules, signs, labels and instructions for use, packaging,
transportation, storage of rigid hysteroscope.
This standard applies to rigid hysteroscope (hereinafter referred to as
hysteroscope). Hysteroscope is mainly used in the medical clinical diagnosis of
uterine cavity disease and treatment together with surgery instruments.
This standard does not apply to high-frequency electric hysteroscope.
2 Normative references
The provisions in following documents become the provisions of this standard
through reference in this standard. For the dated references, the subsequent
amendments (excluding corrections) or revisions do not apply to this standard;
however, parties who reach an agreement based on this standard are
encouraged to study if the latest versions of these documents are applicable.
For undated references, the latest edition of the referenced document applies.
GB/T 191-2000 Packaging - Pictorial marking for handling of goods
GB/T 2829-2002 Sampling procedures and tables for periodic inspection by
attributes (Apply to inspection of process stability)
GB/T 6463-2005 Metallic and other inorganic coatings - Review of methods
of measurement of thickness
GB 9706.1-1995 Medical electrical equipment - Part 1: General
requirements for safety (idt IEC 601-1:1988)
GB 9706.19-2000 Medical electrical equipment - Part 2: Particular
requirements for the safety of endoscopic equipment (idt IEC 60601-2-
18:1996)
GB 11244-2005 General requirements for the medical endoscope and
endoscope accessories
GB/T 14710-1993 The environmental requirements and test methods for
medical electrical equipment
GB/T 16886.1-2001 Biological evaluation of medical devices - Part 1:
Evaluation and testing (idt ISO 10993-1:1997)
GB/T 16886.5-2003 Biological evaluation of medical devices - Part 5: Test
for in vitro cytotoxicity (ISO 10993-5: 1999, IDT)
GB/T 16886.10-2005 Biological evaluation of medical devices - Part 10:
Tests for irritation and delayed-type hypersensitivity (ISO 10993-10:2002,
IDT)
YY 0068 General technical conditions for medical rigid endoscopes
YY 0076-1992 Coating classifications for metal product - Technical
conditions
YY 0466-2003 Medical devices - Symbols to be used with medical device
labels labelling and information to be supplied (ISO 15223:2000, IDT)
3 Classification and composition
3.1 Classification
Hysteroscope may be divided into two types: inspection hysteroscope and
surgery hysteroscope. Surgery hysteroscope is further divided into two types:
integrated and split.
3.2 Composition
3.2.1 The inspection hysteroscope consists of an endoscope, a sheath, an
obturator, a light guide.
3.2.2 The integrated surgery hysteroscope consists of an endoscope with an
instrument channel and a fluid-injection channel as well as a light guide; the
split surgery hysteroscope consists of an endoscope, a sheath, an obturator, a
manipulator, a light guide.
4 Requirements
4.1 Hysteroscope is a rigid endoscope product. In addition to the following
requirements, it shall also comply with the general requirements of YY 0068.
4.2 Surface and edge: The components of the hysteroscope shall be designed
so as not to cause any accidental injury to the human body, all surfaces must
be free of pores, cracks and burrs.
4.3 The basic dimensions of hysteroscope shall meet the requirements of Table
g) For the endoscope whose front end is curved, the curved end shall be firm
and reliable, the outer surface shall be smooth and tidy, there shall be no
light leakage;
h) For the endoscopes that can be sterilized by pressure steam, after the
test, the resolution and illuminance of the endoscope shall be not less than
95% of the original value.
4.6 The sheath and manipulator shall meet the following requirements:
a) The connection of the sheath, the manipulator, the obturator, the
endoscope shall be firm and reliable;
b) Each water-passing valve shall be flexible in rotation, the water-passing
valve and connection part shall be well sealed, the water seepage shall
be not more than five drops in 1 min;
c) The manipulator with the guide plate shall have flexible guiding plates, the
rotation of the rotating hand-wheel shall be flexible and reliable;
d) The endoscope or the instrument channel of the manipulator, the water-
injection channel shall be clean and unobstructed. The instrument channel
shall enable the matching surgery instruments to pass smoothly, the
instrument’s head shall be located in the field of view.
4.7 The flowrate of the hysteroscope’s water injection channel shall not be less
than 130 mL/min.
4.8 The surface of the hysteroscope’s limiting stopper shall be smooth, reliable
to position, easy to move.
4.9 The welding part of the hysteroscope shall be firm and reliable, flat and
smooth, without the phenomenon of de-soldering or surfacing.
4.10 The plating of hysteroscope’s plated components shall comply with the
category-V grade-2 requirements of YY 0076-1992.
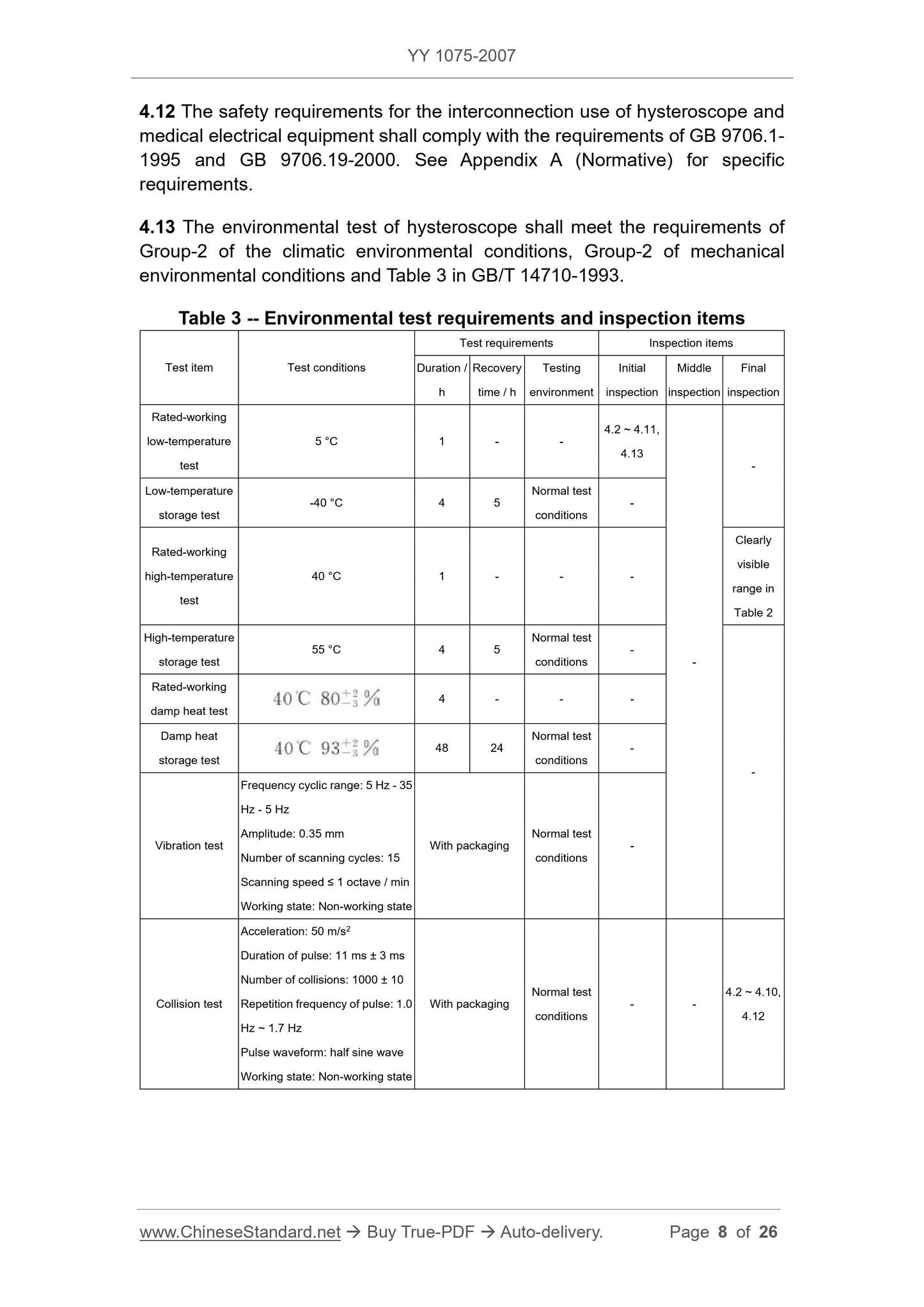
4.11 Hysteroscope is a medical device that is in short-term contact with the
damaged surface. The outer surface material of the insertion part shall be made
of materials that have...
Share