PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1566.1-2017 English PDF (YYT1566.1-2017)
YY/T 1566.1-2017 English PDF (YYT1566.1-2017)
Precio habitual
$150.00 USD
Precio habitual
Precio de oferta
$150.00 USD
Precio unitario
/
por
Los gastos de envío se calculan en la pantalla de pago.
No se pudo cargar la disponibilidad de retiro
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY/T 1566.1-2017
Historical versions: YY/T 1566.1-2017
Preview True-PDF (Reload/Scroll if blank)
YY/T 1566.1-2017: Autologous blood processing devices for single use--Part 1: Blood cell recoery sets, centrifuge bowl type
YY/T 1566.1-2017
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.20
C 31
Autologous blood processing devices for single use -
Part 1. Blood cell recovery sets, centrifuge bowl type
ISSUED ON. JULY 17, 2017
IMPLEMENTED ON. JULY 01, 2018
Issued by. China Food and Drug Administration
Table of Contents
Foreword . 4
Introduction .. 5
1 Scope .. 6
2 Normative references . 6
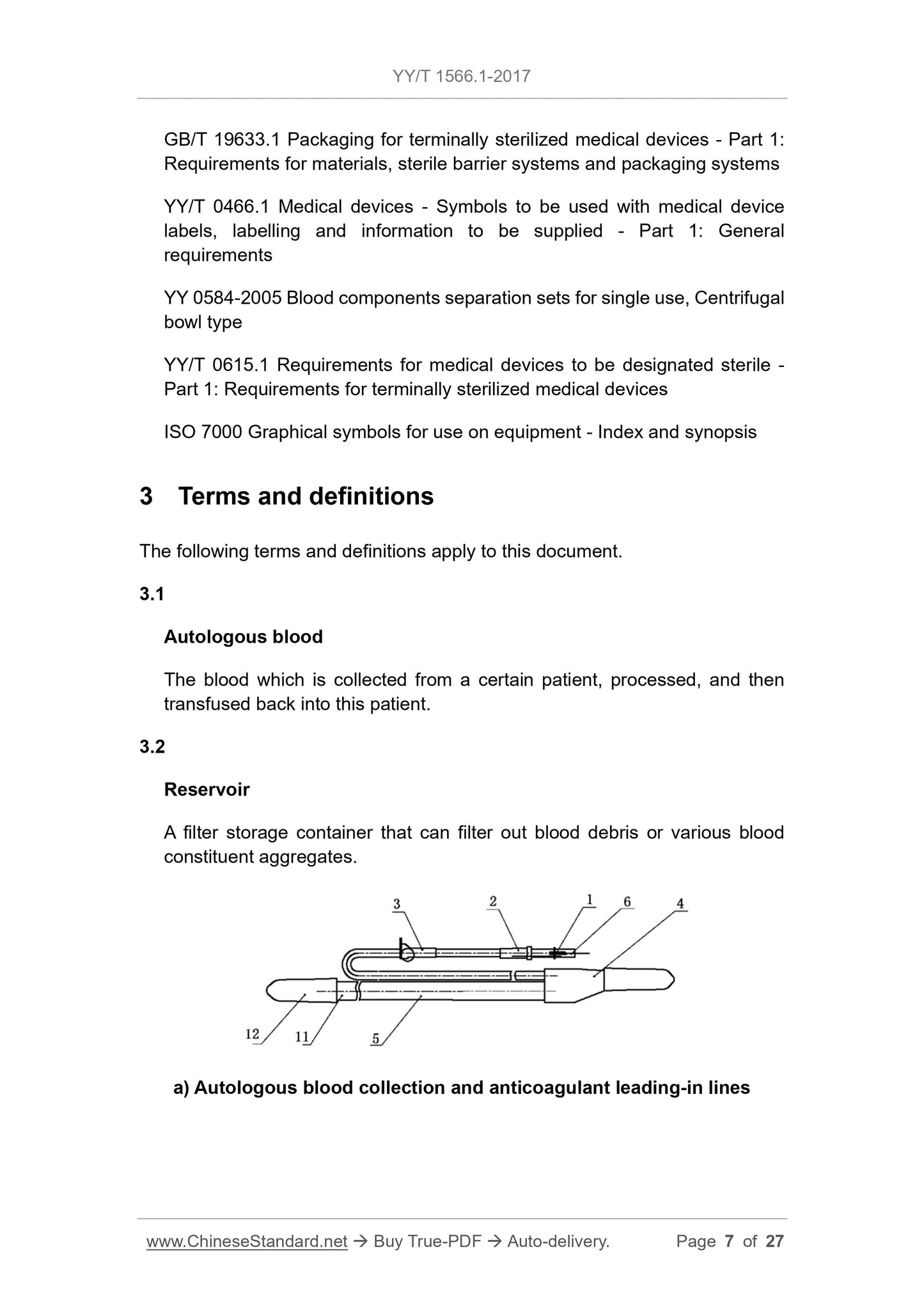
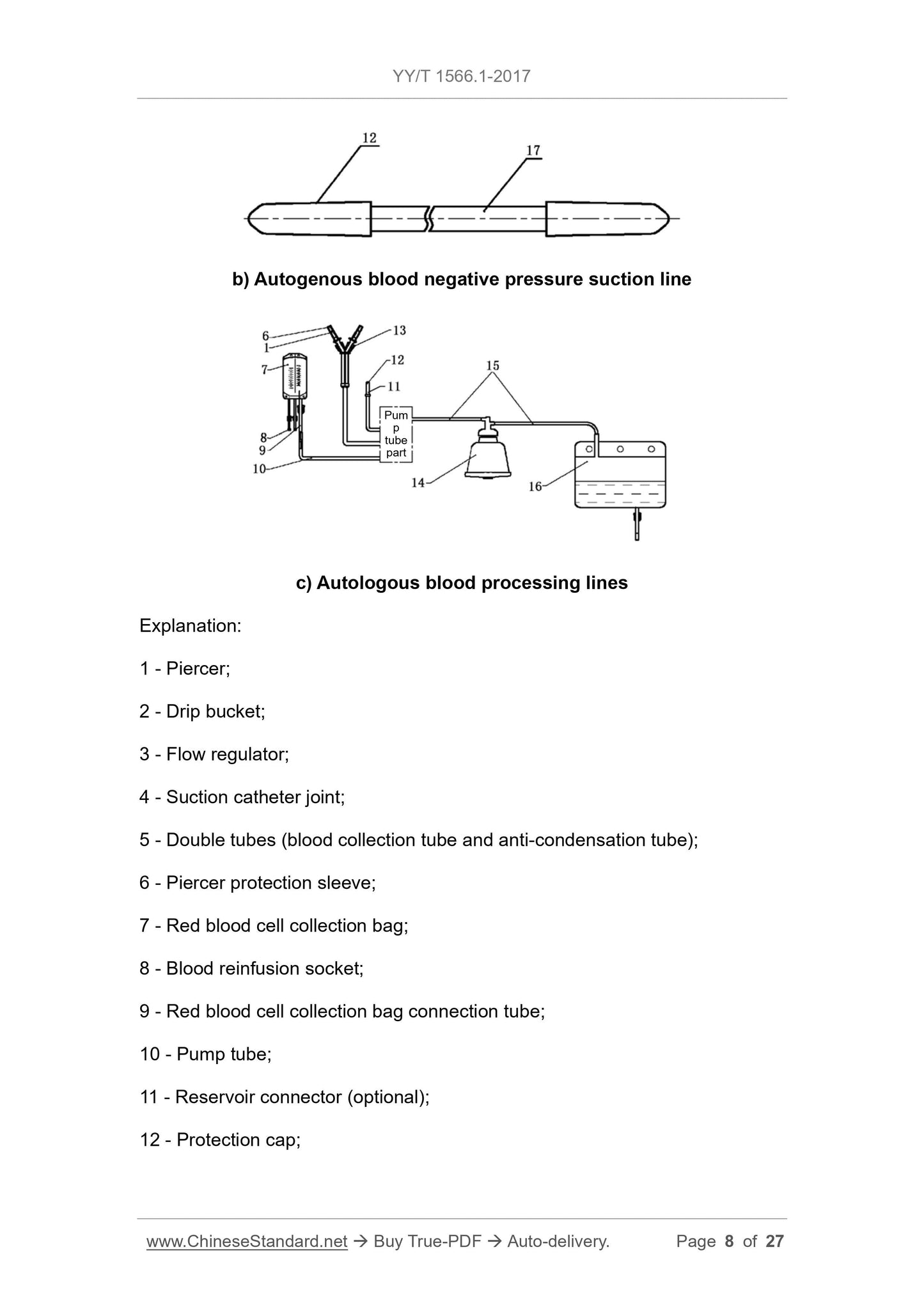
3 Terms and definitions . 7
4 Structure . 9
5 Physical requirements . 9
5.1 Appearance . 9
5.2 Particle contamination . 10
5.2.1 Separation bowl . 10
5.2.2 Pipeline system . 10
5.2.3 Red blood cell collection bag . 10
5.3 Sealing . 10
5.4 Connection strength . 11
5.5 Frictional heat .. 11
5.6 Noise . 11
5.7 Residual amount . 11
5.8 Color scale . 12
5.9 Piercer . 12
5.10 Drip bucket .. 12
5.11 Flow regulator . 12
5.12 Pump tube) elasticity . 12
5.13 Position limit clip) (if any) . 12
5.14 Fixture .. 13
5.15 Interface . 13
5.16 Protective sleeve . 13
5.17 Red blood cell collection bag . 13
6 Chemical requirements . 13
6.1 Separation bowl and pipeline system .. 13
6.1.1 Reducing substances . 13
6.1.2 Metal ions .. 14
6.1.3 pH . 14
6.1.4 Evaporation residue .. 14
6.1.5 UV absorbance . 14
6.2 Red blood cell collection bag .. 14
6.3 Ethylene oxide residues .. 14
7 Biological requirements . 15
7.1 Biological compatibility . 15
7.2 Sterile .. 15
7.3 Bacterial endotoxin .. 15
8 Type test. 15
9 Markings . 15
9.1 General .. 15
9.2 Single package marking .. 16
9.3 Shelf packaging marking . 16
10 Packaging . 17
Appendix A (Informative) Descriptions . 18
Appendix B (Normative) Physical test . 21
Appendix C (Normative) Chemical test . 25
References . 27
Autologous blood processing devices for single use -
Part 1. Blood cell recovery sets, centrifuge bowl type
1 Scope
This part of YY/T 1566 specifies the requirements for the centrifuge bowl type
blood cell recovery set used in surgical operations. It is a single-use product to
ensure the safety of use with autologous blood recovery device.
This part applies to centrifuge bowl type blood cell recovery set that are
intended to be used in conjunction with other autologous blood processing
devices during surgery, and are expected to be used in conjunction with
autologous blood recovery device. It consists of a tube system, a separation
bowl, a red blood cell collection bag, and a waste solution bag. It does not
include a blood collection filter (reservoir).
2 Normative references
The following documents are essential to the application of this document. For
the dated documents, only the versions with the dates indicated are applicable
to this document; for the undated documents, only the latest version (including
all the amendments) are applicable to this standard.
GB/T 6682 Water for analytical laboratory use - Specification and test
methods
GB 8368 Infusion sets for single use, gravity feed
GB 14232.1-2004 Plastics collapsible containers for human blood and blood
component - Part 1. Conventional containers
GB/T 14233.1-2008 Test methods for infusion reinfusion injection equipment
for medical use - Part 1. Chemical analysis methods
GB/T 14233.2 Test methods for infusion, reinfusion, injection equipment for
medical use - Part 2. Biological test methods
GB/T 16886.1 Biological evaluation of medical devices - Part 1. Evaluation
and testing within a risk management process
5.1.5 The interface of the separation bowl, clamps, and other component
surfaces shall be smooth and free from burrs.
5.2 Particle contamination
5.2.1 Separation bowl
When measured in accordance with B.1, the number of particles 15 μm ~ 25
μm in 200 mL eluent shall not exceed 6.00 particles/mL, particles greater than
25 μm shall not exceed 3.00 particles/mL.
5.2.2 Pipeline system
When measured in accordance with B.2, the number of particles 15 μm ~ 25
μm on the surface area per square centimeter shall not exceed 1.00, the
number of particles larger than 25 μm shall not exceed 0.50.
5.2.3 Red blood cell collection bag
The production of red blood cell collection bags shall avoid particle
contamination.
When tested in accordance with the provisions of B.3, red blood cell collection
bag shall have no visible particles.
Note. Work is under way to establish limits on the number and size of particles.
The limits and test methods given in pharmacopoeias (such as the limits and
methods of preparations specified in the European Pharmacopoeia) are
currently available.
5.3 Sealing
5.3.1 When the separation bowl is subject to particle contamination test in
accordance with B.1, the separation bowl shall be free from leakage.
Note. The sealability of the separation bowl and the particle contamination test
are combined.
5.3.2 SEAL the other ends of the pipelines in the pipeline system, LEAVE one
end and LEAD in 50 kPa air pressure for 15 s. There shall be no continuous air
bubble leakage in the underwater test.
6.1.2 Metal ions
When measured by atomic absorption spectrophotometry (AAS) or equivalent,
the total content of antimony, chromium, copper, lead, and tin in the extract shall
not exceed 1 μg/mL, the content of cadmium shall not exceed 0.1 μg/mL.
When tested in accordance with C.3, the color present in the extract shall not
exceed the standard control solution with a mass concentration ρ(Pb2+) = 1
μg/mL.
6.1.3 pH
When tested in accordance with C.4, the difference between the pH of the test
solution prepared in accordance with C.1.3 and the blank solution shall not
exceed 1.5.
6.1.4 Evaporation residue
When tested in accordance with C.5, the total amount of evaporation residue in
50 mL of the test solution prepared in accordance with C.1.3 shall not exceed
2 mg.
6.1.5 UV absorbance
When tested in accordance with C.6, the absorbance of the test solution
prepared in accordance with C.1.3 in the range of 250 nm ~ 320 nm shall not
exceed 0.1.
6.2 Red blood cell collection bag
The test solution prepared in accordance with C.1.4 shall meet the
requirements of GB 14232.1-2004.
6.3 Ethylene oxide residues
If ethylene oxide is used for sterilization, when tested in accordance with
Appendix C, the residual ethylene oxide residuals per set of centrifuge bowl
type blood cell recovery set (excluding the waste solution bag) shall not exceed
4.0 mg.
Appendix A
(Informative)
Descriptions
A.1 Scope
Appendix A of ANSI/AAMI AT6.2013 “Autologous blood reinfusion devices”
describes the clinical application of autologous blood reinfusion as follows.
Autologous blood reinfusion refers to the collection, storage, and reinfusion of
blood products from the same patient. There are many cases where an
autologous blood reinfusion is ideally useful or life-saving, especially when
allogeneic blood is not immediately available. These conditions include the
following types of clinical autologous blood reinfusion.
a) Collection, storage and reinfusion during optional surgery or emergency
during surgery;
b) Collection, storage and reinfusion in the event of a life-threatening injury
or major blood loss.
All autologous blood reinfusion devices will have blood contact with the
surrounding environment, so it will use anticoagulants, purification (filtration),
w...
Get QUOTATION in 1-minute: Click YY/T 1566.1-2017
Historical versions: YY/T 1566.1-2017
Preview True-PDF (Reload/Scroll if blank)
YY/T 1566.1-2017: Autologous blood processing devices for single use--Part 1: Blood cell recoery sets, centrifuge bowl type
YY/T 1566.1-2017
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.20
C 31
Autologous blood processing devices for single use -
Part 1. Blood cell recovery sets, centrifuge bowl type
ISSUED ON. JULY 17, 2017
IMPLEMENTED ON. JULY 01, 2018
Issued by. China Food and Drug Administration
Table of Contents
Foreword . 4
Introduction .. 5
1 Scope .. 6
2 Normative references . 6
3 Terms and definitions . 7
4 Structure . 9
5 Physical requirements . 9
5.1 Appearance . 9
5.2 Particle contamination . 10
5.2.1 Separation bowl . 10
5.2.2 Pipeline system . 10
5.2.3 Red blood cell collection bag . 10
5.3 Sealing . 10
5.4 Connection strength . 11
5.5 Frictional heat .. 11
5.6 Noise . 11
5.7 Residual amount . 11
5.8 Color scale . 12
5.9 Piercer . 12
5.10 Drip bucket .. 12
5.11 Flow regulator . 12
5.12 Pump tube) elasticity . 12
5.13 Position limit clip) (if any) . 12
5.14 Fixture .. 13
5.15 Interface . 13
5.16 Protective sleeve . 13
5.17 Red blood cell collection bag . 13
6 Chemical requirements . 13
6.1 Separation bowl and pipeline system .. 13
6.1.1 Reducing substances . 13
6.1.2 Metal ions .. 14
6.1.3 pH . 14
6.1.4 Evaporation residue .. 14
6.1.5 UV absorbance . 14
6.2 Red blood cell collection bag .. 14
6.3 Ethylene oxide residues .. 14
7 Biological requirements . 15
7.1 Biological compatibility . 15
7.2 Sterile .. 15
7.3 Bacterial endotoxin .. 15
8 Type test. 15
9 Markings . 15
9.1 General .. 15
9.2 Single package marking .. 16
9.3 Shelf packaging marking . 16
10 Packaging . 17
Appendix A (Informative) Descriptions . 18
Appendix B (Normative) Physical test . 21
Appendix C (Normative) Chemical test . 25
References . 27
Autologous blood processing devices for single use -
Part 1. Blood cell recovery sets, centrifuge bowl type
1 Scope
This part of YY/T 1566 specifies the requirements for the centrifuge bowl type
blood cell recovery set used in surgical operations. It is a single-use product to
ensure the safety of use with autologous blood recovery device.
This part applies to centrifuge bowl type blood cell recovery set that are
intended to be used in conjunction with other autologous blood processing
devices during surgery, and are expected to be used in conjunction with
autologous blood recovery device. It consists of a tube system, a separation
bowl, a red blood cell collection bag, and a waste solution bag. It does not
include a blood collection filter (reservoir).
2 Normative references
The following documents are essential to the application of this document. For
the dated documents, only the versions with the dates indicated are applicable
to this document; for the undated documents, only the latest version (including
all the amendments) are applicable to this standard.
GB/T 6682 Water for analytical laboratory use - Specification and test
methods
GB 8368 Infusion sets for single use, gravity feed
GB 14232.1-2004 Plastics collapsible containers for human blood and blood
component - Part 1. Conventional containers
GB/T 14233.1-2008 Test methods for infusion reinfusion injection equipment
for medical use - Part 1. Chemical analysis methods
GB/T 14233.2 Test methods for infusion, reinfusion, injection equipment for
medical use - Part 2. Biological test methods
GB/T 16886.1 Biological evaluation of medical devices - Part 1. Evaluation
and testing within a risk management process
5.1.5 The interface of the separation bowl, clamps, and other component
surfaces shall be smooth and free from burrs.
5.2 Particle contamination
5.2.1 Separation bowl
When measured in accordance with B.1, the number of particles 15 μm ~ 25
μm in 200 mL eluent shall not exceed 6.00 particles/mL, particles greater than
25 μm shall not exceed 3.00 particles/mL.
5.2.2 Pipeline system
When measured in accordance with B.2, the number of particles 15 μm ~ 25
μm on the surface area per square centimeter shall not exceed 1.00, the
number of particles larger than 25 μm shall not exceed 0.50.
5.2.3 Red blood cell collection bag
The production of red blood cell collection bags shall avoid particle
contamination.
When tested in accordance with the provisions of B.3, red blood cell collection
bag shall have no visible particles.
Note. Work is under way to establish limits on the number and size of particles.
The limits and test methods given in pharmacopoeias (such as the limits and
methods of preparations specified in the European Pharmacopoeia) are
currently available.
5.3 Sealing
5.3.1 When the separation bowl is subject to particle contamination test in
accordance with B.1, the separation bowl shall be free from leakage.
Note. The sealability of the separation bowl and the particle contamination test
are combined.
5.3.2 SEAL the other ends of the pipelines in the pipeline system, LEAVE one
end and LEAD in 50 kPa air pressure for 15 s. There shall be no continuous air
bubble leakage in the underwater test.
6.1.2 Metal ions
When measured by atomic absorption spectrophotometry (AAS) or equivalent,
the total content of antimony, chromium, copper, lead, and tin in the extract shall
not exceed 1 μg/mL, the content of cadmium shall not exceed 0.1 μg/mL.
When tested in accordance with C.3, the color present in the extract shall not
exceed the standard control solution with a mass concentration ρ(Pb2+) = 1
μg/mL.
6.1.3 pH
When tested in accordance with C.4, the difference between the pH of the test
solution prepared in accordance with C.1.3 and the blank solution shall not
exceed 1.5.
6.1.4 Evaporation residue
When tested in accordance with C.5, the total amount of evaporation residue in
50 mL of the test solution prepared in accordance with C.1.3 shall not exceed
2 mg.
6.1.5 UV absorbance
When tested in accordance with C.6, the absorbance of the test solution
prepared in accordance with C.1.3 in the range of 250 nm ~ 320 nm shall not
exceed 0.1.
6.2 Red blood cell collection bag
The test solution prepared in accordance with C.1.4 shall meet the
requirements of GB 14232.1-2004.
6.3 Ethylene oxide residues
If ethylene oxide is used for sterilization, when tested in accordance with
Appendix C, the residual ethylene oxide residuals per set of centrifuge bowl
type blood cell recovery set (excluding the waste solution bag) shall not exceed
4.0 mg.
Appendix A
(Informative)
Descriptions
A.1 Scope
Appendix A of ANSI/AAMI AT6.2013 “Autologous blood reinfusion devices”
describes the clinical application of autologous blood reinfusion as follows.
Autologous blood reinfusion refers to the collection, storage, and reinfusion of
blood products from the same patient. There are many cases where an
autologous blood reinfusion is ideally useful or life-saving, especially when
allogeneic blood is not immediately available. These conditions include the
following types of clinical autologous blood reinfusion.
a) Collection, storage and reinfusion during optional surgery or emergency
during surgery;
b) Collection, storage and reinfusion in the event of a life-threatening injury
or major blood loss.
All autologous blood reinfusion devices will have blood contact with the
surrounding environment, so it will use anticoagulants, purification (filtration),
w...
Share