1
/
/
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
GB 1886.304-2020 English PDF (GB1886.304-2020)
GB 1886.304-2020 English PDF (GB1886.304-2020)
Normaalihinta
$125.00 USD
Normaalihinta
Alennushinta
$125.00 USD
Yksikköhinta
/
kohti
Toimituskulut lasketaan kassalla.
Noudon saatavuutta ei voitu ladata
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click GB 1886.304-2020
Historical versions: GB 1886.304-2020
Preview True-PDF (Reload/Scroll if blank)
GB 1886.304-2020: National food safety standard - Food additive - Phosphoric acid (wet process)
GB 1886.304-2020
GB
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National Food Safety Standard - Food Additive -
Phosphoric Acid (wet process)
ISSUED ON: SEPTEMBER 11, 2020
IMPLEMENTED ON: MARCH 11, 2021
Issued by: National Health Commission of the People’s Republic of China;
State Administration for Market Regulation.
Table of Contents
1 Scope ... 3
2 Molecular Formula and Relative Molecular Mass ... 3
3 Technical Requirements ... 3
Appendix A Inspection Method ... 5
National Food Safety Standard - Food Additive -
Phosphoric Acid (wet process)
1 Scope
This Standard is applicable to food additive - phosphoric acid (wet method) obtained
by the purification process of wet-process crude phosphoric acid through impurity
removal, solvent extraction and refining, etc.
2 Molecular Formula and Relative Molecular Mass
2.1 Molecular Formula
H3PO4
2.2 Relative Molecular Mass
97.99 (in accordance with the international relative molecular mass of Year 2016)
3 Technical Requirements
3.1 Sensory Requirements
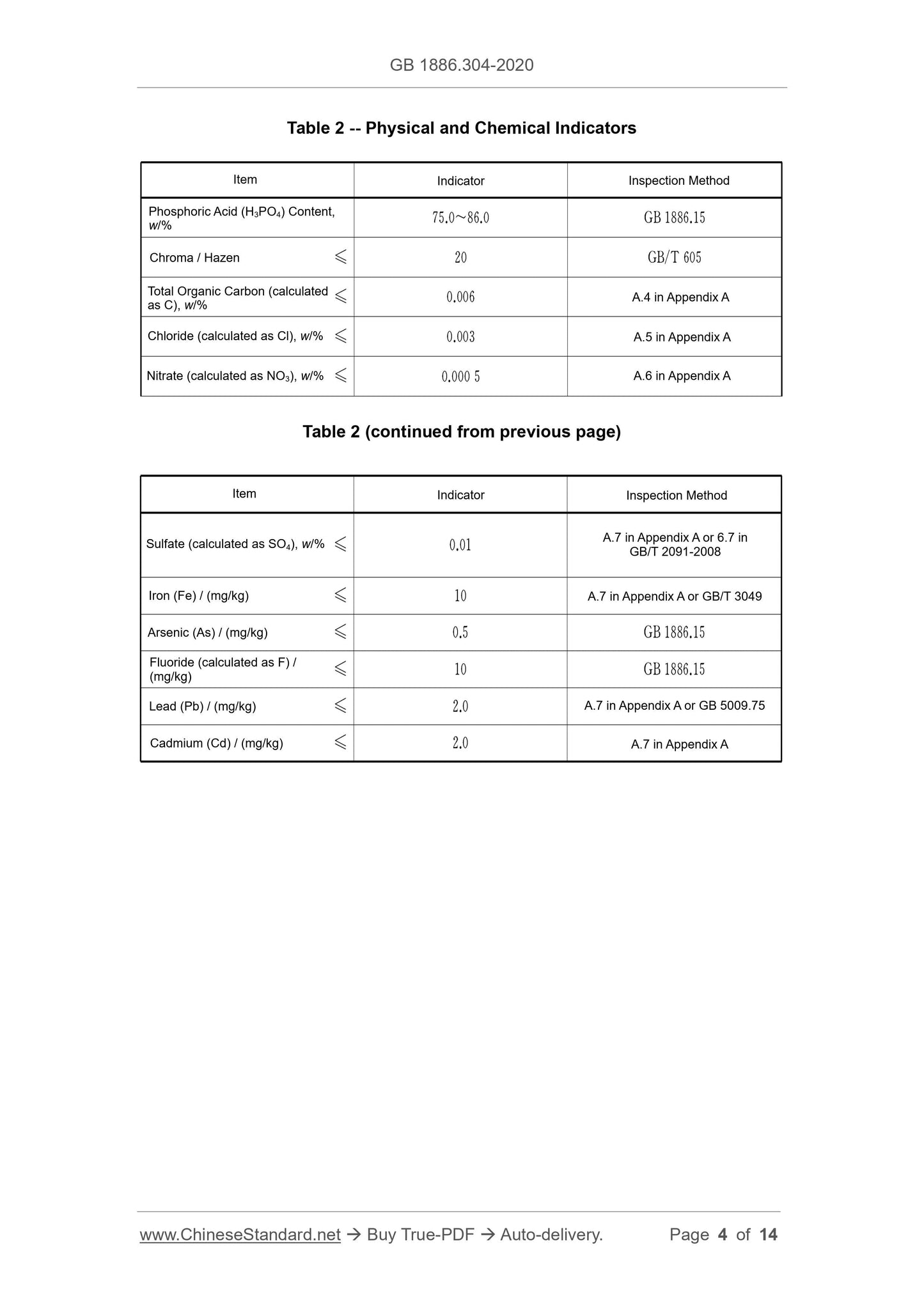
The sensory requirements shall comply with the stipulations of Table 1.
Table 1 -- Sensory Requirements
3.2 Physical and Chemical Indicators
The physical and chemical indicators shall comply with the stipulations of Table 2.
A.4.1.1 Method summary
The sample is under acidic conditions. Purge with nitrogen to remove inorganic carbon.
Through sodium sulfate oxidation, it is quickly generated into carbon dioxide at 100 °C.
Through an infrared analyzer, the content of organic carbon is detected.
A.4.1.2 Reagents and materials
A.4.1.2.1 Sodium persulfate solution: 100 g/L. Weigh-take 100 g of sodium persulfate,
dissolve it in an appropriate amount of carbon dioxide-free water; use carbon dioxide-
free water to dilute it to 1,000 mL.
A.4.1.2.2 Phosphoric acid solution: 5%. Measure-take 59 mL of phosphoric acid; use
carbon dioxide-free water to dilute it to 1,000 mL.
A.4.1.2.3 Total carbon standard stock solution: 1 mL of solution contains 1.0 mg of
carbon (C). Weigh-take 2.1254 g of reference potassium hydrogen phthalate dried at
120 °C ± 2 °C for 2 h. Add carbon dioxide-free water to dissolve it, then, transfer it into
a 1,000 mL volumetric flask. Use carbon dioxide-free water to dilute to the scale, then,
shake it well.
A.4.1.2.4 Total carbon standard service solution: 1 mL of solution contains 0.1 mg of
carbon (C). Use a pipette to transfer-take 10 mL of total carbon standard stock solution
(see A.4.1.2.3); place it in a 100 mL volumetric flask. Use carbon dioxide-free water to
dilute it to the scale, then, shake it well.
A.4.1.2.5 Carbon dioxide-free water: prepare it in accordance with the method
specified in GB/T 603.
A.4.1.3 Instruments and equipment
Total organic carbon (TOC) analyzer: the used high-purity nitrogen complies with the
requirements of GB/T 8979.
A.4.1.4 Analytical procedures
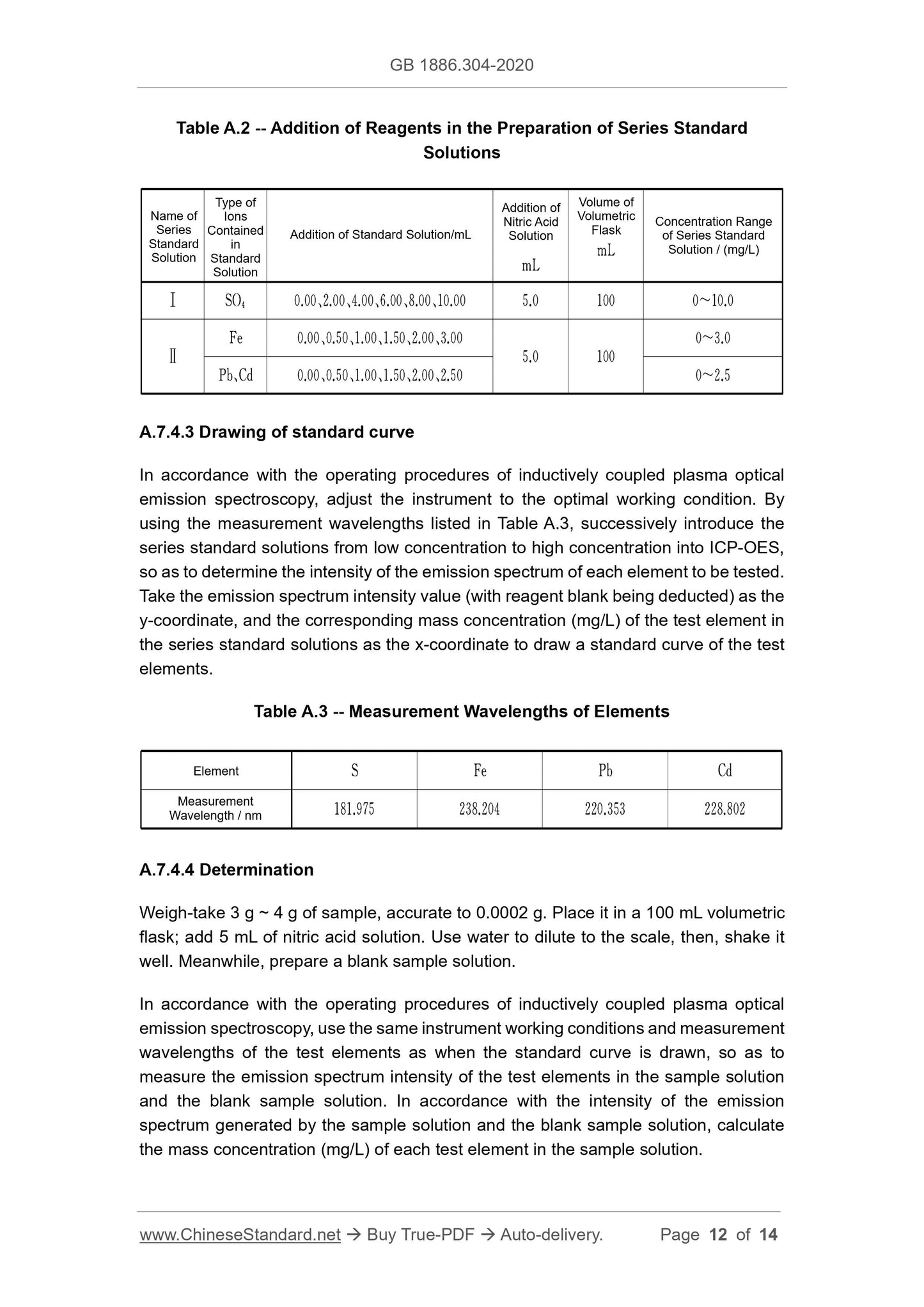
A.4.1.4.1 Drawing of standard curve
Respectively transfer-take 0.00 mL, 1.00 mL, 2.00 mL, 3.00 mL and 4.00 mL of the
total carbon standard service solution; respectively place them in a 100 mL volumetric
flask. Use carbon dioxide-free water to dilute to the scale, then, shake it well. Adjust
the total organic carbon (TOC) analyzer to the optimal test conditions. In accordance
with the sequence of concentration from low to high, successively conduct the
determination. For each standard solution being determined, the instrument
automatically inhales 5.00 mL of corresponding standard solution, 0.50 mL of
phosphoric acid solution and 1.00 mL of sodium persulfate solution. After the reaction
is completed, the instrument automatically measures the peak area of the standard
carbon (IC).
A.4.2.2 Reagents and materials
A.4.2.2.1 Total carbon standard stock solution: 1 mL of solution contains 1.0 mg of
carbon (C). Weigh-take 2.1254 g of reference potassium hydrogen phthalate dried at
120 °C ± 2 °C for 2 h. Add carbon dioxide-free water to dissolve it, then, transfer it into
a 1,000 mL volumetric flask. Use carbon dioxide-free water to dilute to the scale, then,
shake it well.
A.4.2.2.2 Total carbon standard service solution: 1 mL of solution contains 0.1 mg of
carbon (C). Use a pipette to transfer-take 10 mL of total carbon standard stock solution
(see A.4.2.2.1), place it in a 100 mL volumetric flask. Use carbon dioxide-free water to
dilute to the scale, then, shake it well.
A.4.2.2.3 Inorganic carbon standard stock solution: 1 mL of solution contains 1.0 mg
of carbon (C). Weigh-take 3.50 g of sodium bicarbonate previously dried in a silica gel
drier for 2 h, and 4.41 g of reference sodium carbonate dried at 280 °C ~ 290 °C for 1
h. Add carbon dioxide-free water to dissolve it, then, transfer it into a 1,000 mL
volumetric flask. Use carbon dioxide-free water to dilute to the scale, then, shake it
well.
A.4.2.2.4 Inorganic carbon standard service solution: 1 mL of solution contains 0.01
mg of carbon (C). Use a pipette to transfer-take 1.00 mL of the inorganic carbon
standard stock solution (see A.4.2.2.3); place it in a 100 mL volumetric flask. Use
carbon dioxide-free water to dilute to the scale, then, shake it well.
A.4.2.2.5 Carbon dioxide-free water: prepare it in accordance with the method
specified in GB/T 603.
A.4.2.3 Instruments and equipment
Total organic carbon (TOC) analyzer: the used high-purity oxygen complies with the
requirements of GB/T 14599.
A.4.2.4 Analytical procedures
A.4.2.4.1 Drawing of standard curve
Respectively transfer-take 0.00 mL, 1.00 mL, 2.00 mL, 3.00 mL and 4.00 mL of the
total carbon standard service solution; respectively place them in a 100 mL volumetric
flask. Use carbon dioxide-free water to dilute to the scale, then, shake it well. Adjust
the total organic carbon (TOC) analyzer to the optimal test conditions. Draw the
standard solution to measure the peak area. Subtract the peak area of the blank
solution from the peak area of each standard solution. Take the mass concentration
(mg/L) of carbon as the x-coordinate, and the corresponding peak area as the y-
coordinate to draw a total carbon standard curve.
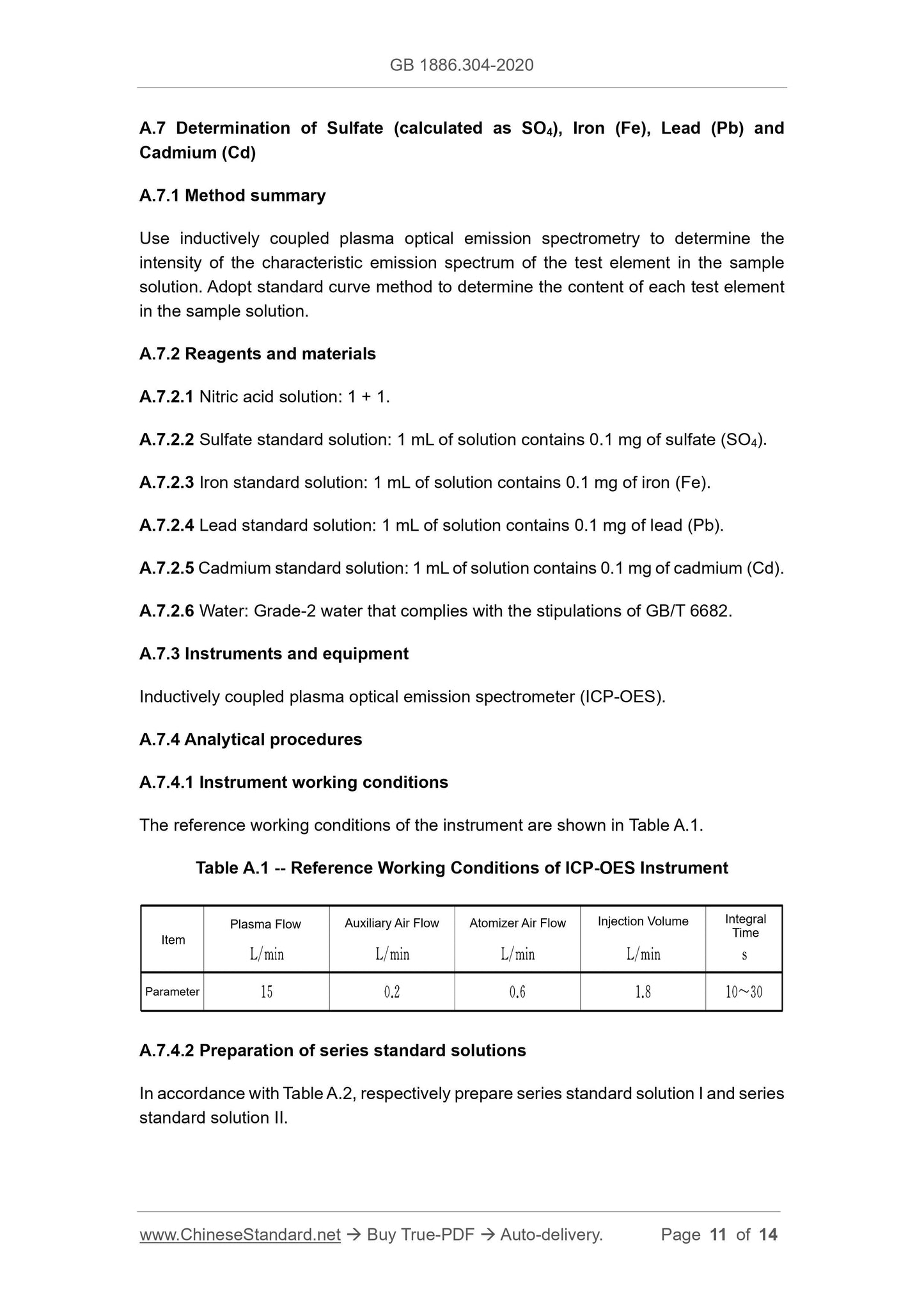
A.5.1 Method summary
In the nitric acid medium, the chloride in the sample and the added silver nitrate
generate silver chloride precipitate. Through the comparison with the standard turbidity
solution, determine the chloride content in the sample.
A.5.2 Reagents and materials
A.5.2.1 Nitric acid solution: 1 + 2.
A.5.2.2 Silver nitrate solution: 17 g/L.
A.5.2.3 Chloride standard solution: 1 mL of solution contains 0.010 mg of chlorine (Cl).
Use a pipette to transfer-take 10.00 mL of the chloride standard solution prepared in
accordance with GB/T 602; place it in a 100 mL volumetric flask. Use water to dilute to
the scale, then, shake it well. This solution shall be prepared right before use.
A.5.3 Analytical procedures
Weigh-take 5.00 g ± 0.01 g of sample; place it in a 25 mL colorimetric tube. Add water
to a volume of about 20 mL. Then, successively add 2 mL of nitric acid solution and 1
mL of silver nitrate solution. Use water to dilute to the scale, then, shake it well. Place
it for 10 min, then, compare it with the standard turbidity solution. The turbidity of the
sample solution shall not be greater than the standard turbidity solution.
The preparation of standard turbidity solution: use a pipette to transfer-take 15.00 mL
of chloride standard solution; place it in a 25 mL of colorimetric tube. Process it in the
same mode and at t...
Get QUOTATION in 1-minute: Click GB 1886.304-2020
Historical versions: GB 1886.304-2020
Preview True-PDF (Reload/Scroll if blank)
GB 1886.304-2020: National food safety standard - Food additive - Phosphoric acid (wet process)
GB 1886.304-2020
GB
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National Food Safety Standard - Food Additive -
Phosphoric Acid (wet process)
ISSUED ON: SEPTEMBER 11, 2020
IMPLEMENTED ON: MARCH 11, 2021
Issued by: National Health Commission of the People’s Republic of China;
State Administration for Market Regulation.
Table of Contents
1 Scope ... 3
2 Molecular Formula and Relative Molecular Mass ... 3
3 Technical Requirements ... 3
Appendix A Inspection Method ... 5
National Food Safety Standard - Food Additive -
Phosphoric Acid (wet process)
1 Scope
This Standard is applicable to food additive - phosphoric acid (wet method) obtained
by the purification process of wet-process crude phosphoric acid through impurity
removal, solvent extraction and refining, etc.
2 Molecular Formula and Relative Molecular Mass
2.1 Molecular Formula
H3PO4
2.2 Relative Molecular Mass
97.99 (in accordance with the international relative molecular mass of Year 2016)
3 Technical Requirements
3.1 Sensory Requirements
The sensory requirements shall comply with the stipulations of Table 1.
Table 1 -- Sensory Requirements
3.2 Physical and Chemical Indicators
The physical and chemical indicators shall comply with the stipulations of Table 2.
A.4.1.1 Method summary
The sample is under acidic conditions. Purge with nitrogen to remove inorganic carbon.
Through sodium sulfate oxidation, it is quickly generated into carbon dioxide at 100 °C.
Through an infrared analyzer, the content of organic carbon is detected.
A.4.1.2 Reagents and materials
A.4.1.2.1 Sodium persulfate solution: 100 g/L. Weigh-take 100 g of sodium persulfate,
dissolve it in an appropriate amount of carbon dioxide-free water; use carbon dioxide-
free water to dilute it to 1,000 mL.
A.4.1.2.2 Phosphoric acid solution: 5%. Measure-take 59 mL of phosphoric acid; use
carbon dioxide-free water to dilute it to 1,000 mL.
A.4.1.2.3 Total carbon standard stock solution: 1 mL of solution contains 1.0 mg of
carbon (C). Weigh-take 2.1254 g of reference potassium hydrogen phthalate dried at
120 °C ± 2 °C for 2 h. Add carbon dioxide-free water to dissolve it, then, transfer it into
a 1,000 mL volumetric flask. Use carbon dioxide-free water to dilute to the scale, then,
shake it well.
A.4.1.2.4 Total carbon standard service solution: 1 mL of solution contains 0.1 mg of
carbon (C). Use a pipette to transfer-take 10 mL of total carbon standard stock solution
(see A.4.1.2.3); place it in a 100 mL volumetric flask. Use carbon dioxide-free water to
dilute it to the scale, then, shake it well.
A.4.1.2.5 Carbon dioxide-free water: prepare it in accordance with the method
specified in GB/T 603.
A.4.1.3 Instruments and equipment
Total organic carbon (TOC) analyzer: the used high-purity nitrogen complies with the
requirements of GB/T 8979.
A.4.1.4 Analytical procedures
A.4.1.4.1 Drawing of standard curve
Respectively transfer-take 0.00 mL, 1.00 mL, 2.00 mL, 3.00 mL and 4.00 mL of the
total carbon standard service solution; respectively place them in a 100 mL volumetric
flask. Use carbon dioxide-free water to dilute to the scale, then, shake it well. Adjust
the total organic carbon (TOC) analyzer to the optimal test conditions. In accordance
with the sequence of concentration from low to high, successively conduct the
determination. For each standard solution being determined, the instrument
automatically inhales 5.00 mL of corresponding standard solution, 0.50 mL of
phosphoric acid solution and 1.00 mL of sodium persulfate solution. After the reaction
is completed, the instrument automatically measures the peak area of the standard
carbon (IC).
A.4.2.2 Reagents and materials
A.4.2.2.1 Total carbon standard stock solution: 1 mL of solution contains 1.0 mg of
carbon (C). Weigh-take 2.1254 g of reference potassium hydrogen phthalate dried at
120 °C ± 2 °C for 2 h. Add carbon dioxide-free water to dissolve it, then, transfer it into
a 1,000 mL volumetric flask. Use carbon dioxide-free water to dilute to the scale, then,
shake it well.
A.4.2.2.2 Total carbon standard service solution: 1 mL of solution contains 0.1 mg of
carbon (C). Use a pipette to transfer-take 10 mL of total carbon standard stock solution
(see A.4.2.2.1), place it in a 100 mL volumetric flask. Use carbon dioxide-free water to
dilute to the scale, then, shake it well.
A.4.2.2.3 Inorganic carbon standard stock solution: 1 mL of solution contains 1.0 mg
of carbon (C). Weigh-take 3.50 g of sodium bicarbonate previously dried in a silica gel
drier for 2 h, and 4.41 g of reference sodium carbonate dried at 280 °C ~ 290 °C for 1
h. Add carbon dioxide-free water to dissolve it, then, transfer it into a 1,000 mL
volumetric flask. Use carbon dioxide-free water to dilute to the scale, then, shake it
well.
A.4.2.2.4 Inorganic carbon standard service solution: 1 mL of solution contains 0.01
mg of carbon (C). Use a pipette to transfer-take 1.00 mL of the inorganic carbon
standard stock solution (see A.4.2.2.3); place it in a 100 mL volumetric flask. Use
carbon dioxide-free water to dilute to the scale, then, shake it well.
A.4.2.2.5 Carbon dioxide-free water: prepare it in accordance with the method
specified in GB/T 603.
A.4.2.3 Instruments and equipment
Total organic carbon (TOC) analyzer: the used high-purity oxygen complies with the
requirements of GB/T 14599.
A.4.2.4 Analytical procedures
A.4.2.4.1 Drawing of standard curve
Respectively transfer-take 0.00 mL, 1.00 mL, 2.00 mL, 3.00 mL and 4.00 mL of the
total carbon standard service solution; respectively place them in a 100 mL volumetric
flask. Use carbon dioxide-free water to dilute to the scale, then, shake it well. Adjust
the total organic carbon (TOC) analyzer to the optimal test conditions. Draw the
standard solution to measure the peak area. Subtract the peak area of the blank
solution from the peak area of each standard solution. Take the mass concentration
(mg/L) of carbon as the x-coordinate, and the corresponding peak area as the y-
coordinate to draw a total carbon standard curve.
A.5.1 Method summary
In the nitric acid medium, the chloride in the sample and the added silver nitrate
generate silver chloride precipitate. Through the comparison with the standard turbidity
solution, determine the chloride content in the sample.
A.5.2 Reagents and materials
A.5.2.1 Nitric acid solution: 1 + 2.
A.5.2.2 Silver nitrate solution: 17 g/L.
A.5.2.3 Chloride standard solution: 1 mL of solution contains 0.010 mg of chlorine (Cl).
Use a pipette to transfer-take 10.00 mL of the chloride standard solution prepared in
accordance with GB/T 602; place it in a 100 mL volumetric flask. Use water to dilute to
the scale, then, shake it well. This solution shall be prepared right before use.
A.5.3 Analytical procedures
Weigh-take 5.00 g ± 0.01 g of sample; place it in a 25 mL colorimetric tube. Add water
to a volume of about 20 mL. Then, successively add 2 mL of nitric acid solution and 1
mL of silver nitrate solution. Use water to dilute to the scale, then, shake it well. Place
it for 10 min, then, compare it with the standard turbidity solution. The turbidity of the
sample solution shall not be greater than the standard turbidity solution.
The preparation of standard turbidity solution: use a pipette to transfer-take 15.00 mL
of chloride standard solution; place it in a 25 mL of colorimetric tube. Process it in the
same mode and at t...
Share