1
/
de
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
GB 1886.214-2016 English PDF (GB1886.214-2016)
GB 1886.214-2016 English PDF (GB1886.214-2016)
Prix habituel
$85.00 USD
Prix habituel
Prix promotionnel
$85.00 USD
Prix unitaire
/
par
Frais d'expédition calculés à l'étape de paiement.
Impossible de charger la disponibilité du service de retrait
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click GB 1886.214-2016
Historical versions: GB 1886.214-2016
Preview True-PDF (Reload/Scroll if blank)
GB 1886.214-2016: Food additive -- Calcium carbonate
GB 1886.214-2016
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National Food Safety Standard -
Food additive - Calcium carbonate
(including light and heavy calcium carbonate)
ISSUED ON: AUGUST 31, 2016
IMPLEMENTED ON: JANUARY 01, 2017
Issued by: National Health and Family Planning commission of PRC
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Molecular formula and relative molecular mass ... 4
3 Technical requirements ... 4
Appendix A Testing method ... 6
National Food Safety Standard -
Food additive - Calcium carbonate
(including light and heavy calcium carbonate)
1 Scope
This standard applies to food additives light calcium carbonate, crushed
limestone, calcite as made through precipitation as well as the food additive
heavy calcium carbonate as made from oyster shell.
2 Molecular formula and relative molecular mass
2.1 Molecular formula
CaCO3
2.2 Relative molecular mass
100.09 (according to the international relative atomic mass in 2013)
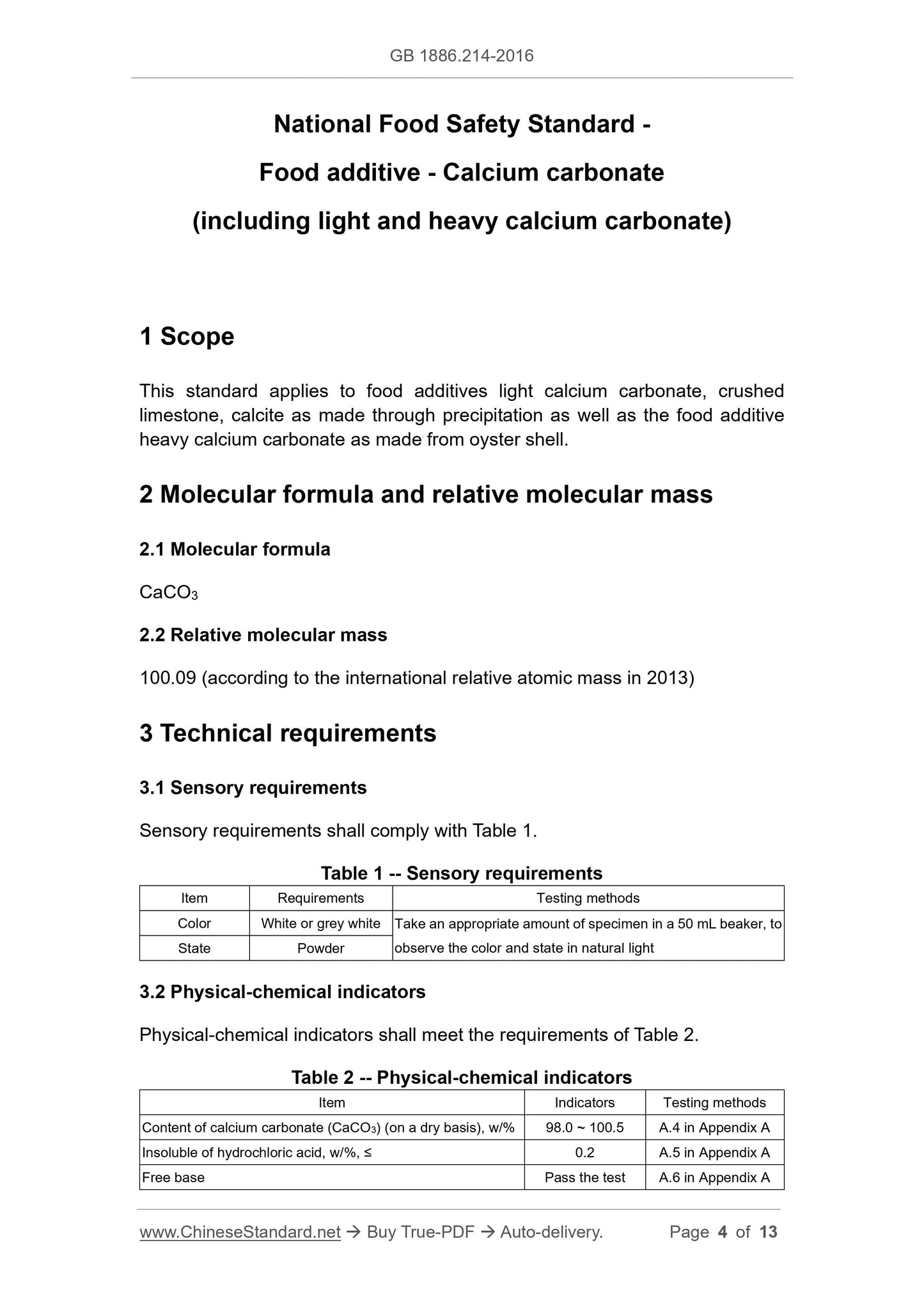
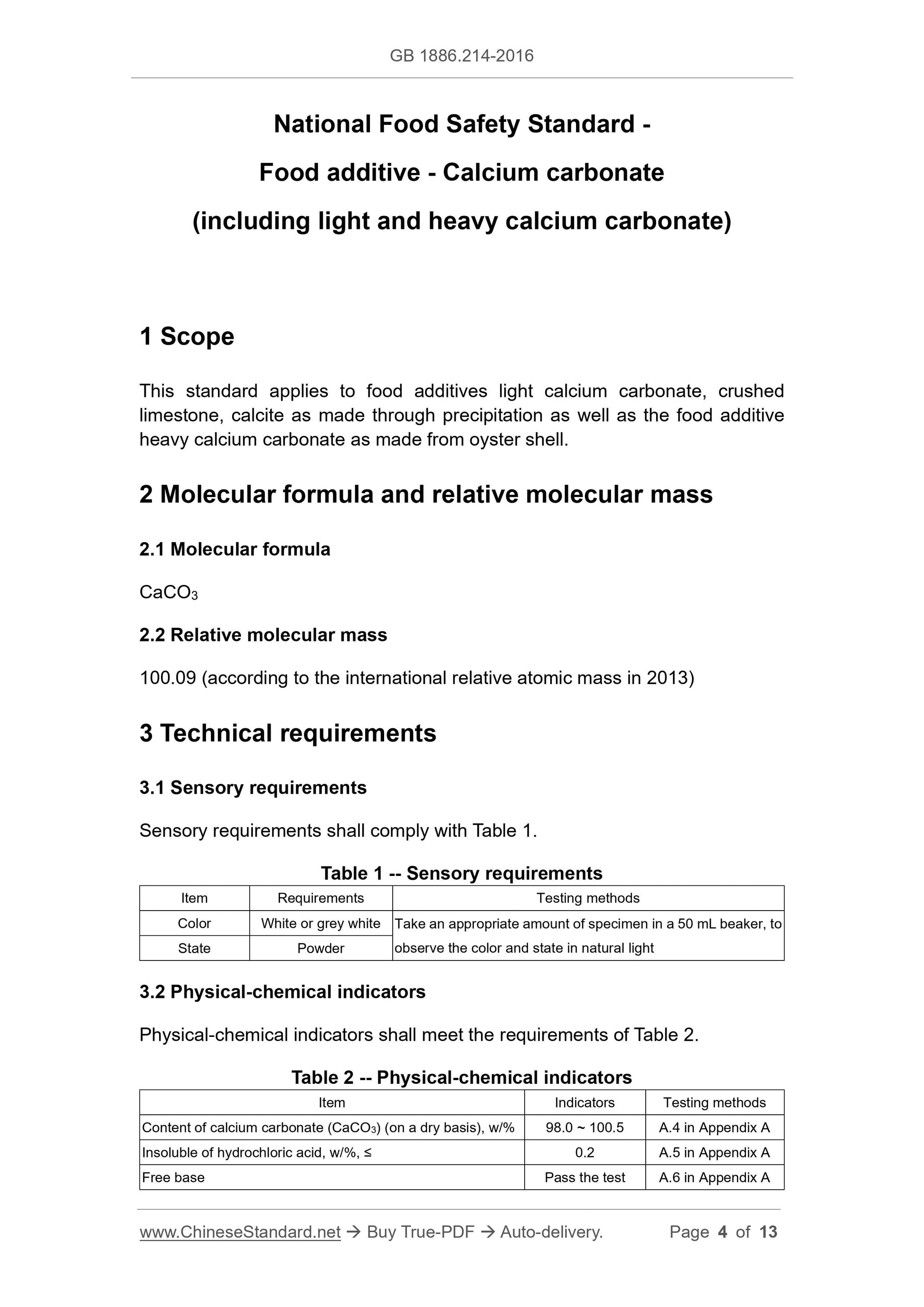
3 Technical requirements
3.1 Sensory requirements
Sensory requirements shall comply with Table 1.
Table 1 -- Sensory requirements
Item Requirements Testing methods
Color White or grey white Take an appropriate amount of specimen in a 50 mL beaker, to
observe the color and state in natural light State Powder
3.2 Physical-chemical indicators
Physical-chemical indicators shall meet the requirements of Table 2.
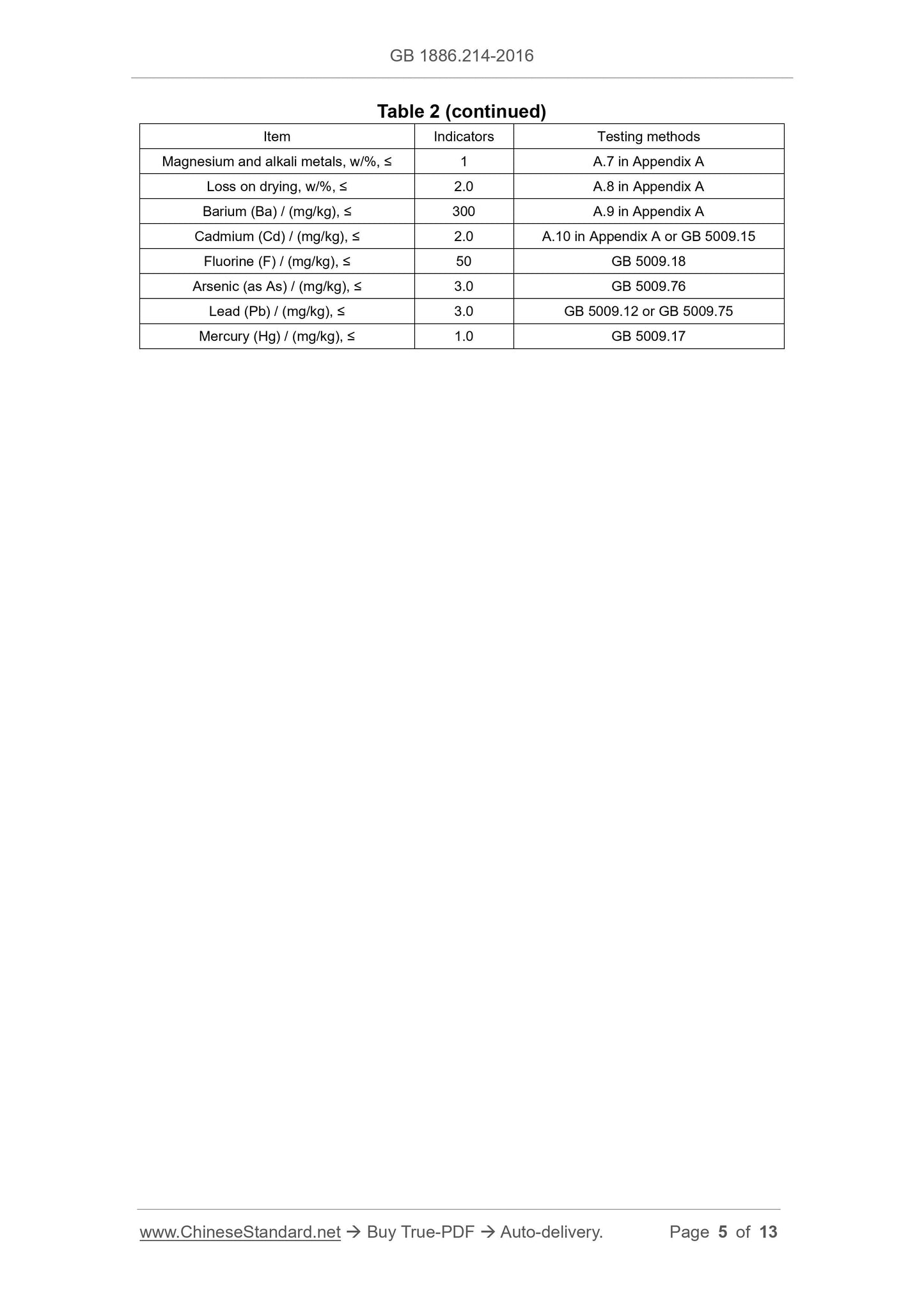
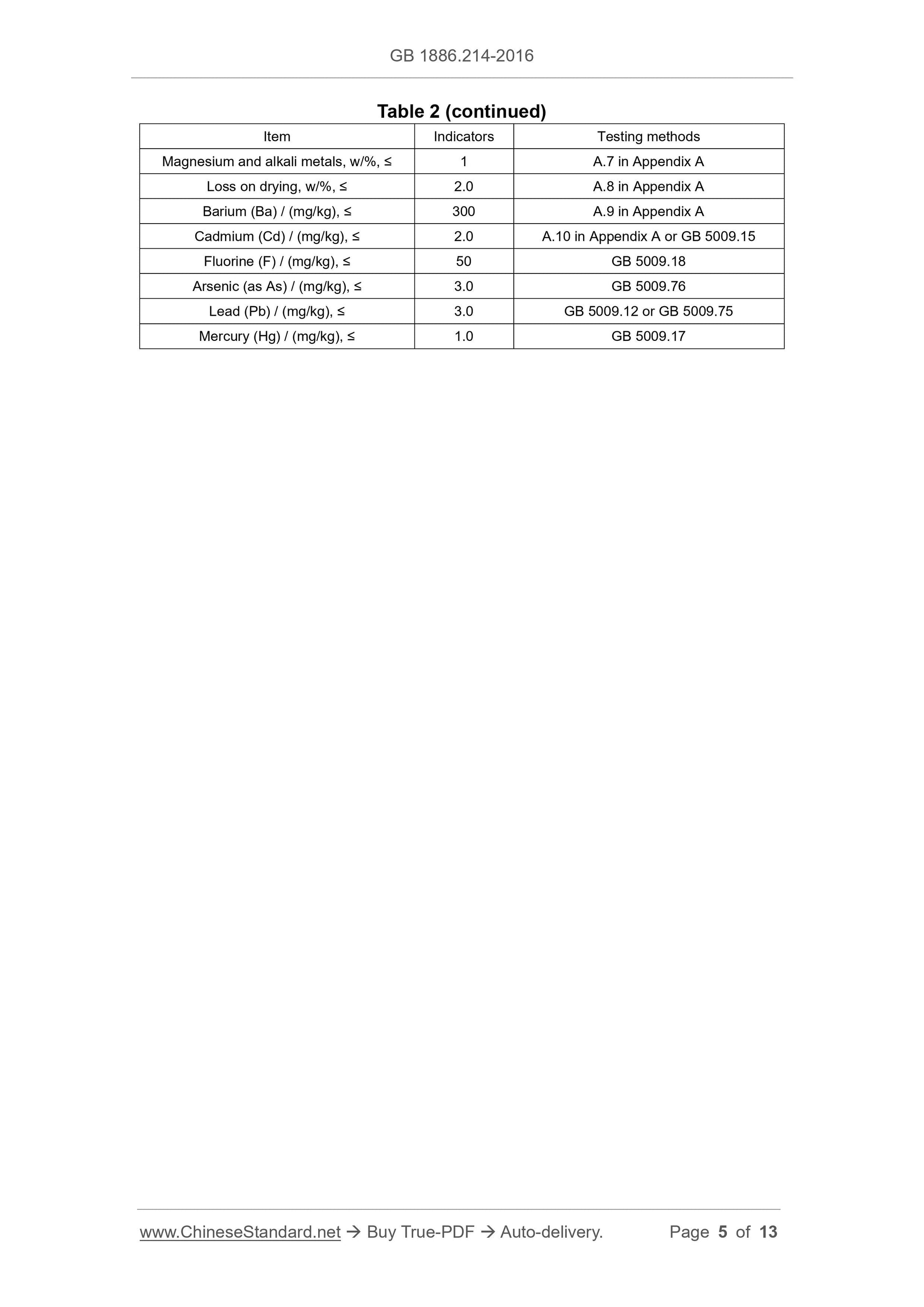
Table 2 -- Physical-chemical indicators
Item Indicators Testing methods
Content of calcium carbonate (CaCO3) (on a dry basis), w/% 98.0 ~ 100.5 A.4 in Appendix A
Insoluble of hydrochloric acid, w/%, ≤ 0.2 A.5 in Appendix A
Free base Pass the test A.6 in Appendix A
Appendix A
Testing method
A.1 Warning
Some of the reagents used in the test methods of this standard are toxic or
corrosive, so during operation, it shall take appropriate safety and protective
measures.
A.2 General requirements
The reagents and water used in this standard refer to, when no other
requirements are indicated, the analytical pure reagents and the grade-3 water
as specified in GB/T 6682. The standard solution, standard solution for
determination of impurities, reagents and preparations as required in the test of
this standard, when no other requirements are indicated, shall be prepared
according to the provisions of GB/T 601, GB/T 602, GB/T 603. When the
reagents which are used to prepare the solution used are not specified, the
solution refers to aqueous solution.
A.3 Identification test
A.3.1 Identification of calcium
Take a small amount of specimen. Add hydrochloric acid solution (1 + 2) to
dissolve it. Use phenolphthalein solution (10 g/L) as an indicator solution. Use
ammonia solution (1 + 3) to adjust to neutral. Add ammonium oxalate solution
(35 g/L) to produce a white precipitate, which is soluble in the hydrochloric acid
solution (1 + 2) but insoluble in glacial acetic acid.
A.3.2 Identification of carbonate
Take a small amount of specimen. Add hydrochloric acid solution (1 + 2) to
generate a gas. Lead this gas into calcium hydroxide solution (3 g/L). A white
precipitate is produced.
A.4 Determination of calcium carbonate (CaCO3) content (on a dry basis)
A.4.1 Reagents and materials
A.4.1.1 Hydrochloric acid solution: 1 + 1.
A.4.1.2 Sodium hydroxide solution: 100 g/L.
A.4.1.3 Triethanolamine solution: 1 + 3.
(CaCO3) = 100.09];
m1 - Mass of the specimen, in grams (g);
25 - Volume of the specimen solution as pipetted, in milliliters (mL);
250 - Volume of the volumetric flask, in milliliters (mL);
1000 - Conversion factor.
The test results are based on the arithmetic mean of the parallel determination
results. The absolute difference between the two independent determinations
as obtained under repetitive conditions is not more than 0.2%.
A.5 Determination of hydrochloric acid insoluble
A.5.1 Reagents and materials
A.5.1.1 Hydrochloric acid solution: 1 + 1.
A.5.1.2 Silver nitrate solution: 10 g/L.
A.5.2 Instruments and equipment
High-temperature furnace: It can control the temperature at 800 °C ± 25 °C.
A.5.3 Analytical procedures
Weigh approximately 5 g of the specimen, accurate to 0.01 g. Place it in a tall
beaker. After adding water to wet it, slowly add 25 mL of hydrochloric acid
solution. Heat to boil it. When it is still hot, use medium-speed quantitative filter
paper to filter it. Use hot water to rinse the beaker. Rinse the filter paper until
the filtrate has no chloride ions (use silver nitrate solution to check it). Transfer
the filter paper together with the insoluble into a porcelain crucible which had
been burned at 800 °C ± 25 °C to a constant mass. After ashing it on an electric
furnace, burn it at 800 °C ± 25 °C until the mass is constant.
A.5.4 Calculation of results
The mass fraction of hydrochloric acid insoluble, w2, is calculated according to
formula (A.2):
Where:
m3 - The mass of hydrochloric acid insoluble and crucible, in grams (g);
In a water bath, heat it for 1 h. After cooling it, transfer it all into a 100 mL
volumetric flask. Add water to the mark. Shake it uniformly. Place it overnight.
Perform dry filtration. Pipette 50 mL of the filtrate. Place it in a porcelain crucible
that has been burned at 800 °C ± 25 °C to a constant mass. Add 0.5 mL of
sulfuric acid. Evaporate it dry. Burn it at 800 °C ± 25 °C until the mass is constant.
A.7.4 Calculation of results
The mass fraction of magnesium and alkali metal content, w3, is calculated
according to formula (A.3):
Where:
m5 - The mass of the crucible and residue, in grams (g);
m6 - The mass of the empty crucible, in grams (g);
m7 - The mass of the specimen, in grams (g);
50 - The volume of the specimen solution as pipetted, in milliliters (mL);
100 - The volume of the volumetric flask, in milliliters (mL).
The test results are based on the arithmetic mean of the parallel determination
results. The absolute difference between the two independent determinations
as obtained under repetitive conditions is not more than 0.2%.
A.8 Determination of loss on drying
A.8.1 Instruments and equipment
A.8.1.1 Electrothermal drying oven: The temperature can be controlled at
200 °C ± 5 °C.
A.8.1.2 Weighing bottle: Φ40 mm x 25 mm.
A.8.2 Analytical procedures
Use a weighing bottle which had been dried to constant weight at 200 °C ± 5 °C
to weigh about 2 g of the specimen, accurate to 0.0002 g. Dry it at 200 °C ±
5 °C for 4 h. Cool it to room temperature. Weigh it, accurate to 0.0002 g.
A.8.3 Calculation of results
The mass fraction of the loss on drying, w4, is calculated according to the
A.10.1 Summary of method
After the specimen is treated, the cadmium ions are led into the atomic
absorption spectrometer in the acidic solution. After atomization, the 228.8 nm
resonance line is absorbed, the absorption amount is proportional to the
cadmium content, which is compared with the standard series for quantification.
A.10.2 Reagents and materials
A.10.2.1 Hydrochloric acid solution: 1 + 4.
A.10.2.2 Cadmium standard solution: 1 mL solution contains 0.001 mg of
cadmium (Cd). Pipette 1 mL of the cadmium standard solution as prepared in
GB/T 602. Transfer it to a 100 mL volumetric flask. Add water to the mark. Shake
it uniformly. Prepare it before use.
A.10.3 Instruments and equipment
Atomic absorption spectrophotometer.
A.10.4 Analytical procedures
A.10.4.1 Preparation of...
Get QUOTATION in 1-minute: Click GB 1886.214-2016
Historical versions: GB 1886.214-2016
Preview True-PDF (Reload/Scroll if blank)
GB 1886.214-2016: Food additive -- Calcium carbonate
GB 1886.214-2016
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National Food Safety Standard -
Food additive - Calcium carbonate
(including light and heavy calcium carbonate)
ISSUED ON: AUGUST 31, 2016
IMPLEMENTED ON: JANUARY 01, 2017
Issued by: National Health and Family Planning commission of PRC
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Molecular formula and relative molecular mass ... 4
3 Technical requirements ... 4
Appendix A Testing method ... 6
National Food Safety Standard -
Food additive - Calcium carbonate
(including light and heavy calcium carbonate)
1 Scope
This standard applies to food additives light calcium carbonate, crushed
limestone, calcite as made through precipitation as well as the food additive
heavy calcium carbonate as made from oyster shell.
2 Molecular formula and relative molecular mass
2.1 Molecular formula
CaCO3
2.2 Relative molecular mass
100.09 (according to the international relative atomic mass in 2013)
3 Technical requirements
3.1 Sensory requirements
Sensory requirements shall comply with Table 1.
Table 1 -- Sensory requirements
Item Requirements Testing methods
Color White or grey white Take an appropriate amount of specimen in a 50 mL beaker, to
observe the color and state in natural light State Powder
3.2 Physical-chemical indicators
Physical-chemical indicators shall meet the requirements of Table 2.
Table 2 -- Physical-chemical indicators
Item Indicators Testing methods
Content of calcium carbonate (CaCO3) (on a dry basis), w/% 98.0 ~ 100.5 A.4 in Appendix A
Insoluble of hydrochloric acid, w/%, ≤ 0.2 A.5 in Appendix A
Free base Pass the test A.6 in Appendix A
Appendix A
Testing method
A.1 Warning
Some of the reagents used in the test methods of this standard are toxic or
corrosive, so during operation, it shall take appropriate safety and protective
measures.
A.2 General requirements
The reagents and water used in this standard refer to, when no other
requirements are indicated, the analytical pure reagents and the grade-3 water
as specified in GB/T 6682. The standard solution, standard solution for
determination of impurities, reagents and preparations as required in the test of
this standard, when no other requirements are indicated, shall be prepared
according to the provisions of GB/T 601, GB/T 602, GB/T 603. When the
reagents which are used to prepare the solution used are not specified, the
solution refers to aqueous solution.
A.3 Identification test
A.3.1 Identification of calcium
Take a small amount of specimen. Add hydrochloric acid solution (1 + 2) to
dissolve it. Use phenolphthalein solution (10 g/L) as an indicator solution. Use
ammonia solution (1 + 3) to adjust to neutral. Add ammonium oxalate solution
(35 g/L) to produce a white precipitate, which is soluble in the hydrochloric acid
solution (1 + 2) but insoluble in glacial acetic acid.
A.3.2 Identification of carbonate
Take a small amount of specimen. Add hydrochloric acid solution (1 + 2) to
generate a gas. Lead this gas into calcium hydroxide solution (3 g/L). A white
precipitate is produced.
A.4 Determination of calcium carbonate (CaCO3) content (on a dry basis)
A.4.1 Reagents and materials
A.4.1.1 Hydrochloric acid solution: 1 + 1.
A.4.1.2 Sodium hydroxide solution: 100 g/L.
A.4.1.3 Triethanolamine solution: 1 + 3.
(CaCO3) = 100.09];
m1 - Mass of the specimen, in grams (g);
25 - Volume of the specimen solution as pipetted, in milliliters (mL);
250 - Volume of the volumetric flask, in milliliters (mL);
1000 - Conversion factor.
The test results are based on the arithmetic mean of the parallel determination
results. The absolute difference between the two independent determinations
as obtained under repetitive conditions is not more than 0.2%.
A.5 Determination of hydrochloric acid insoluble
A.5.1 Reagents and materials
A.5.1.1 Hydrochloric acid solution: 1 + 1.
A.5.1.2 Silver nitrate solution: 10 g/L.
A.5.2 Instruments and equipment
High-temperature furnace: It can control the temperature at 800 °C ± 25 °C.
A.5.3 Analytical procedures
Weigh approximately 5 g of the specimen, accurate to 0.01 g. Place it in a tall
beaker. After adding water to wet it, slowly add 25 mL of hydrochloric acid
solution. Heat to boil it. When it is still hot, use medium-speed quantitative filter
paper to filter it. Use hot water to rinse the beaker. Rinse the filter paper until
the filtrate has no chloride ions (use silver nitrate solution to check it). Transfer
the filter paper together with the insoluble into a porcelain crucible which had
been burned at 800 °C ± 25 °C to a constant mass. After ashing it on an electric
furnace, burn it at 800 °C ± 25 °C until the mass is constant.
A.5.4 Calculation of results
The mass fraction of hydrochloric acid insoluble, w2, is calculated according to
formula (A.2):
Where:
m3 - The mass of hydrochloric acid insoluble and crucible, in grams (g);
In a water bath, heat it for 1 h. After cooling it, transfer it all into a 100 mL
volumetric flask. Add water to the mark. Shake it uniformly. Place it overnight.
Perform dry filtration. Pipette 50 mL of the filtrate. Place it in a porcelain crucible
that has been burned at 800 °C ± 25 °C to a constant mass. Add 0.5 mL of
sulfuric acid. Evaporate it dry. Burn it at 800 °C ± 25 °C until the mass is constant.
A.7.4 Calculation of results
The mass fraction of magnesium and alkali metal content, w3, is calculated
according to formula (A.3):
Where:
m5 - The mass of the crucible and residue, in grams (g);
m6 - The mass of the empty crucible, in grams (g);
m7 - The mass of the specimen, in grams (g);
50 - The volume of the specimen solution as pipetted, in milliliters (mL);
100 - The volume of the volumetric flask, in milliliters (mL).
The test results are based on the arithmetic mean of the parallel determination
results. The absolute difference between the two independent determinations
as obtained under repetitive conditions is not more than 0.2%.
A.8 Determination of loss on drying
A.8.1 Instruments and equipment
A.8.1.1 Electrothermal drying oven: The temperature can be controlled at
200 °C ± 5 °C.
A.8.1.2 Weighing bottle: Φ40 mm x 25 mm.
A.8.2 Analytical procedures
Use a weighing bottle which had been dried to constant weight at 200 °C ± 5 °C
to weigh about 2 g of the specimen, accurate to 0.0002 g. Dry it at 200 °C ±
5 °C for 4 h. Cool it to room temperature. Weigh it, accurate to 0.0002 g.
A.8.3 Calculation of results
The mass fraction of the loss on drying, w4, is calculated according to the
A.10.1 Summary of method
After the specimen is treated, the cadmium ions are led into the atomic
absorption spectrometer in the acidic solution. After atomization, the 228.8 nm
resonance line is absorbed, the absorption amount is proportional to the
cadmium content, which is compared with the standard series for quantification.
A.10.2 Reagents and materials
A.10.2.1 Hydrochloric acid solution: 1 + 4.
A.10.2.2 Cadmium standard solution: 1 mL solution contains 0.001 mg of
cadmium (Cd). Pipette 1 mL of the cadmium standard solution as prepared in
GB/T 602. Transfer it to a 100 mL volumetric flask. Add water to the mark. Shake
it uniformly. Prepare it before use.
A.10.3 Instruments and equipment
Atomic absorption spectrophotometer.
A.10.4 Analytical procedures
A.10.4.1 Preparation of...
Share