1
/
de
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 0654-2017 English PDF (YYT0654-2017)
YY/T 0654-2017 English PDF (YYT0654-2017)
Prix habituel
$145.00 USD
Prix habituel
Prix promotionnel
$145.00 USD
Prix unitaire
/
par
Frais d'expédition calculés à l'étape de paiement.
Impossible de charger la disponibilité du service de retrait
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY/T 0654-2017
Historical versions: YY/T 0654-2017
Preview True-PDF (Reload/Scroll if blank)
YY/T 0654-2017: Automatic chemistry analyzer
YY/T 0654-2017
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.100
C 44
Replacing YY/T 0654-2008
Automatic chemistry analyzer
ISSUED ON. MARCH 28, 2017
IMPLEMENTED ON. APRIL 1, 2018
Issued by. China Food and Drug Administration of China
Table of Contents
Foreword ... 3
1 Scope ... 5
2 Normative references ... 5
3 Terms and definitions ... 6
4 Classification ... 6
5 Requirements ... 7
6 Test methods ... 9
7 Marks and instructions for use ... 15
8 Packaging, transport and storage ... 15
Annex A (normative) Preparation method of 50g/L sodium nitrite solution ... 17
Annex B (informative) Density of pure water at different temperatures at
standard atmospheric pressure ... 18
Foreword
This Standard was drafted in accordance with the rules given in GB/T 1.1-2009.
This Standard was revised based on YY/T 0654-2008. Compared with YY/T
0654-2008, in addition to the editorial modifications, the main technical changes
are as follows.
- modified the applicable scope as automatic biochemical analyzer for
quantitative analysis of various samples by ultraviolet-visible
spectrophotometry;
- in normative references, modified GB/T 14710 “The environmental
requirements and test methods for medical electrical equipment” to GB/T
14710 “Medical electrical environment requirements and test methods.’
- deleted GB/T 2829 “Sampling procedures and tables for periodic inspection
by attributes (Apply to inspection of process stability)” from normative
references;
- deleted YY 0466 “Medical devices - Symbols to be used with medical device
labels labelling and information to be supplied” (ISO 15233.2000, IDT)
from normative references;
- modified the sample carryover rate as not exceeding 0.1% and modified its
test methods (see 5.8, 6.7);
- adjusted the UREA (urea) test range in the intra-assay precision of clinical
projects to 7.0 mmol/L ~ 11.0 mmol/L (see 5.10);
- added the requirements and test methods for applicable provisions of GB
4793.9 and YY 0648 in safety requirements (see 5.13, 6.12);
- added GB/T 18268.1, GB/T 18268.26 electromagnetic compatibility
requirements and test methods (see 5.14, 6.13);
- in the absorbance stability test method, the potassium dichromate solution
used at a wavelength of 340 nm was changed to a yellow orange G
solution (see 6.4);
- in the absorbance repeatability test method, the potassium dichromate
solution used at a wavelength of 340 nm was changed to a yellow orange
G solution (see 6.5);
- the accuracy and repeatability of the test method is modified by the
manufacturer to choose one of two methods (see 6.8);
Automatic chemistry analyzer
1 Scope
This Standard specifies the terms and definitions, classification, requirements,
test methods, marks and instructions for use, packaging, transport and storage
of automatic chemistry analyzer (hereinafter referred to as the analyzer).
This Standard is applicable to automatic biochemical analyzer for quantitative
analysis of various samples by UV-visible spectrophotometry.
2 Normative references
The following referenced documents are indispensable for the application of
this document. For dated references, only the edition cited applies. For undated
references, the latest edition of the referenced document (including any
amendments) applies.
GB/T 191, Packaging and storage marks
GB 4793.1, Safety requirements for electrical equipment for measurement,
control, and laboratory use - Part 1. General requirements
GB 4793.9, Safety requirements for electrical equipment for measurement,
control and laboratory use - Part 9. Particular requirements for automatic and
semi-automatic laboratory equipment for analysis and other purposes
GB/T 14710, Environmental requirement and test methods for medical
electrical equipment
GB/T 18268.1, Electrical equipment for measurement, control and laboratory
use - EMC requirements - Part 1. General requirements
GB/T 18268.26, Electrical equipment for measurement, control and
laboratory use - EMC requirements - Part 26. Particular requirements - In
vitro diagnostic (IVD) medical equipment
GB/T 29791.3, In vitro diagnostic medical devices - Information supplied by
the manufacturer (labelling) - Part 3. In vitro diagnostic instruments for
professional use
YY 0648, Safety requirements for electrical equipment for measurement
control and laboratory use - Part 2-101. Particular requirements for in vitro
Recycling or single use.
5 Requirements
5.1 Normal working environment
5.1.1 Power supply voltage. 220V ± 22V, 50Hz ± 1Hz.
5.1.2 Ambient temperature. 15°C~30°C.
5.1.3 Relative humidity. 40%~85%.
5.1.4 Atmospheric pressure. 86.0kPa~106.0kPa.
NOTE. If the conditions in 5.1.2~5.1.4 are inconsistent with the conditions stated in the manufacturing
mark, the conditions specified in the product shall prevail.
5.2 Stray light
Absorbance is not less than 2.3.
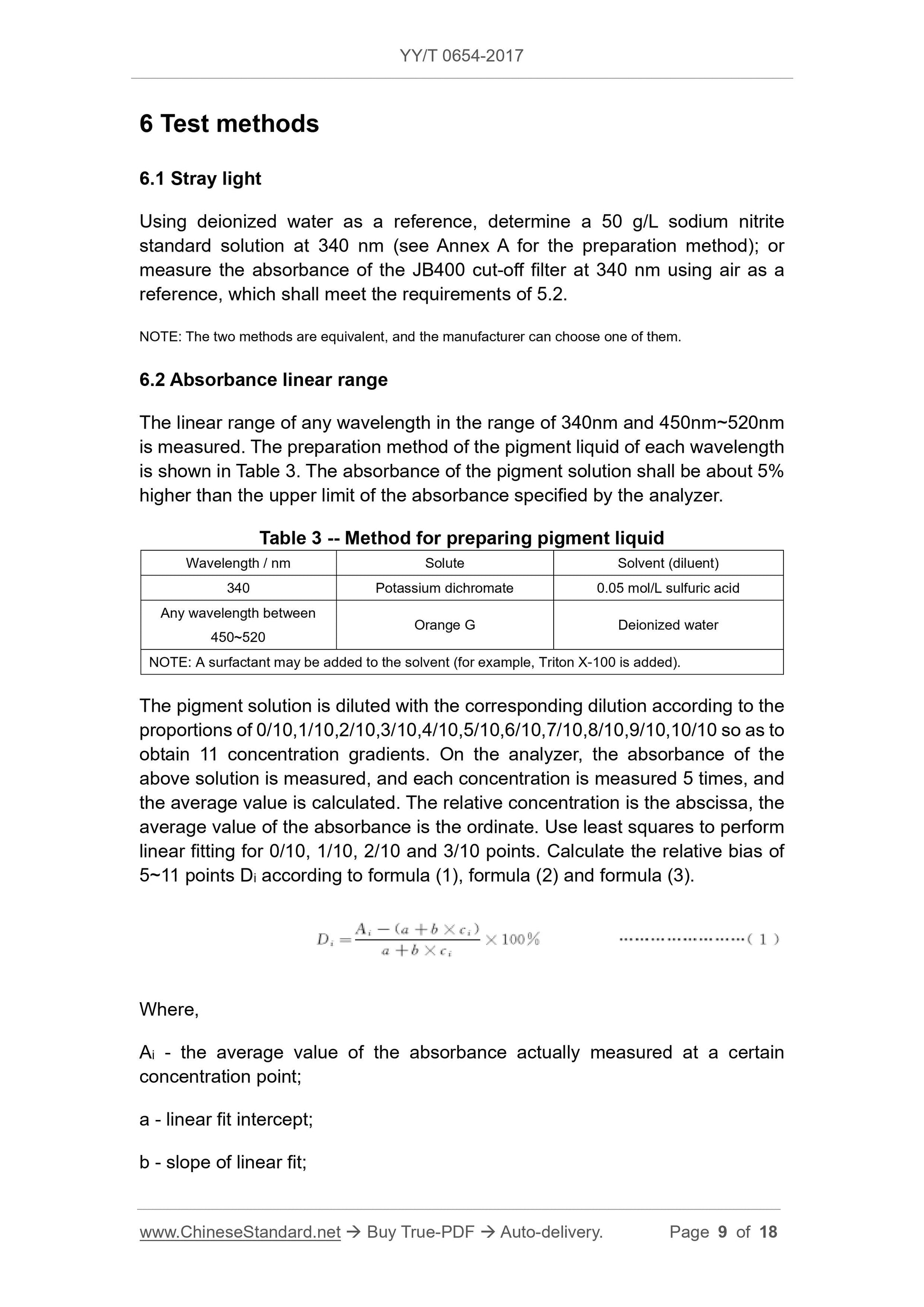
5.3 Absorbance linear range
The maximum absorbance of the relative bias within ±5% shall be no less than
2.0.
5.4 Absorbance accuracy
Comply with Table 1.
Table 1 -- Absorbance accuracy requirements
Absorbance value Allowable error
0.5 ±0.025
1.0 ±0.07
5.5 Absorbance stability
The change in absorbance shall not be greater than 0.01.
5.6 Absorbance repeatability
Expressed by coefficient of variation, not exceeding 1.5%.
5.7 Temperature accuracy and volatility
The temperature value is within ±0.3°C of the set value, and the fluctuation is
not more than ±0.2°C.
5.8 Sample carry-over rate
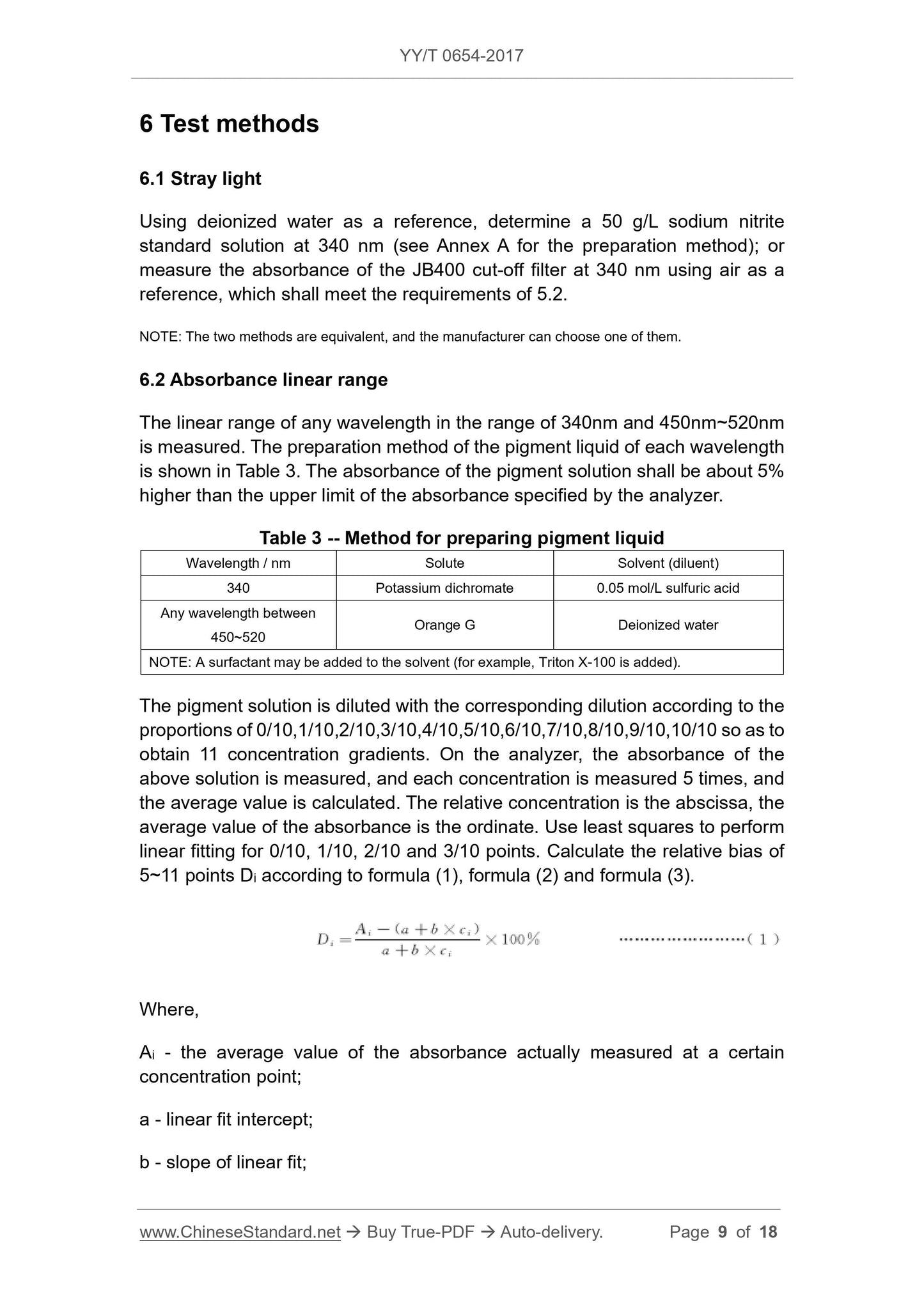
ci - relative concentration;
i - concentration serial number, range is 5~11.
Where,
Ai - the average value of the absorbance actually measured at a certain
concentration point;
ci - relative concentration;
n - selected number of concentration;
i - concentration serial number, range is 1~4.
The absorbance range of relative bias less than ±5% is the linear range of
absorbance and shall meet the requirements of 5.3.
6.3 Absorbance accuracy
Use deionized water as a reference. Determine the absorbance of the
potassium dichromate standard solution of which the absorbance is about 0.5
(with deionized water as blank, allowable deviation is ±5%) and 1.0 (with
deionized water as blank, allowable deviation of ±5%) at 340 nm on the analyzer.
Repeat the measurement 3 times and calculate the difference between the
arithmetic mean of the 3 measurements and the standard value, which shall
meet the requirements of Table 1.
6.4 Absorbance stability
Perform the absorbance stability measurement at any wavelength in the 340
nm and 600 nm to 700 nm wavelength range of the analyzer. The 340 nm
measurement solution is Orange G standard solution with an absorbance of 0.5
(with deionized water as a blank and an allowable deviation of ±5%). The
measurement solution at any wavelength in the wavelength range of 600 nm to
700 nm has an absorbance of 0.5 (in terms of absorbance of 0.5). Deionized
water is blank, allowing a deviation of ±5%) of copper sulfate standard solution.
According to the following setting conditions a) and b), the absorbance of the
above solution shall be measured on the analyzer, and the difference between
Vr - reagent addition volume.
NOTE 1. Surfactants can be added to deionized water (such as TritonX-100).
NOTE 2. Allow adoption of 600nm~700nm as a sub-wavelength.
6.8 Sample loading accuracy and repeatabi...
Get QUOTATION in 1-minute: Click YY/T 0654-2017
Historical versions: YY/T 0654-2017
Preview True-PDF (Reload/Scroll if blank)
YY/T 0654-2017: Automatic chemistry analyzer
YY/T 0654-2017
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.100
C 44
Replacing YY/T 0654-2008
Automatic chemistry analyzer
ISSUED ON. MARCH 28, 2017
IMPLEMENTED ON. APRIL 1, 2018
Issued by. China Food and Drug Administration of China
Table of Contents
Foreword ... 3
1 Scope ... 5
2 Normative references ... 5
3 Terms and definitions ... 6
4 Classification ... 6
5 Requirements ... 7
6 Test methods ... 9
7 Marks and instructions for use ... 15
8 Packaging, transport and storage ... 15
Annex A (normative) Preparation method of 50g/L sodium nitrite solution ... 17
Annex B (informative) Density of pure water at different temperatures at
standard atmospheric pressure ... 18
Foreword
This Standard was drafted in accordance with the rules given in GB/T 1.1-2009.
This Standard was revised based on YY/T 0654-2008. Compared with YY/T
0654-2008, in addition to the editorial modifications, the main technical changes
are as follows.
- modified the applicable scope as automatic biochemical analyzer for
quantitative analysis of various samples by ultraviolet-visible
spectrophotometry;
- in normative references, modified GB/T 14710 “The environmental
requirements and test methods for medical electrical equipment” to GB/T
14710 “Medical electrical environment requirements and test methods.’
- deleted GB/T 2829 “Sampling procedures and tables for periodic inspection
by attributes (Apply to inspection of process stability)” from normative
references;
- deleted YY 0466 “Medical devices - Symbols to be used with medical device
labels labelling and information to be supplied” (ISO 15233.2000, IDT)
from normative references;
- modified the sample carryover rate as not exceeding 0.1% and modified its
test methods (see 5.8, 6.7);
- adjusted the UREA (urea) test range in the intra-assay precision of clinical
projects to 7.0 mmol/L ~ 11.0 mmol/L (see 5.10);
- added the requirements and test methods for applicable provisions of GB
4793.9 and YY 0648 in safety requirements (see 5.13, 6.12);
- added GB/T 18268.1, GB/T 18268.26 electromagnetic compatibility
requirements and test methods (see 5.14, 6.13);
- in the absorbance stability test method, the potassium dichromate solution
used at a wavelength of 340 nm was changed to a yellow orange G
solution (see 6.4);
- in the absorbance repeatability test method, the potassium dichromate
solution used at a wavelength of 340 nm was changed to a yellow orange
G solution (see 6.5);
- the accuracy and repeatability of the test method is modified by the
manufacturer to choose one of two methods (see 6.8);
Automatic chemistry analyzer
1 Scope
This Standard specifies the terms and definitions, classification, requirements,
test methods, marks and instructions for use, packaging, transport and storage
of automatic chemistry analyzer (hereinafter referred to as the analyzer).
This Standard is applicable to automatic biochemical analyzer for quantitative
analysis of various samples by UV-visible spectrophotometry.
2 Normative references
The following referenced documents are indispensable for the application of
this document. For dated references, only the edition cited applies. For undated
references, the latest edition of the referenced document (including any
amendments) applies.
GB/T 191, Packaging and storage marks
GB 4793.1, Safety requirements for electrical equipment for measurement,
control, and laboratory use - Part 1. General requirements
GB 4793.9, Safety requirements for electrical equipment for measurement,
control and laboratory use - Part 9. Particular requirements for automatic and
semi-automatic laboratory equipment for analysis and other purposes
GB/T 14710, Environmental requirement and test methods for medical
electrical equipment
GB/T 18268.1, Electrical equipment for measurement, control and laboratory
use - EMC requirements - Part 1. General requirements
GB/T 18268.26, Electrical equipment for measurement, control and
laboratory use - EMC requirements - Part 26. Particular requirements - In
vitro diagnostic (IVD) medical equipment
GB/T 29791.3, In vitro diagnostic medical devices - Information supplied by
the manufacturer (labelling) - Part 3. In vitro diagnostic instruments for
professional use
YY 0648, Safety requirements for electrical equipment for measurement
control and laboratory use - Part 2-101. Particular requirements for in vitro
Recycling or single use.
5 Requirements
5.1 Normal working environment
5.1.1 Power supply voltage. 220V ± 22V, 50Hz ± 1Hz.
5.1.2 Ambient temperature. 15°C~30°C.
5.1.3 Relative humidity. 40%~85%.
5.1.4 Atmospheric pressure. 86.0kPa~106.0kPa.
NOTE. If the conditions in 5.1.2~5.1.4 are inconsistent with the conditions stated in the manufacturing
mark, the conditions specified in the product shall prevail.
5.2 Stray light
Absorbance is not less than 2.3.
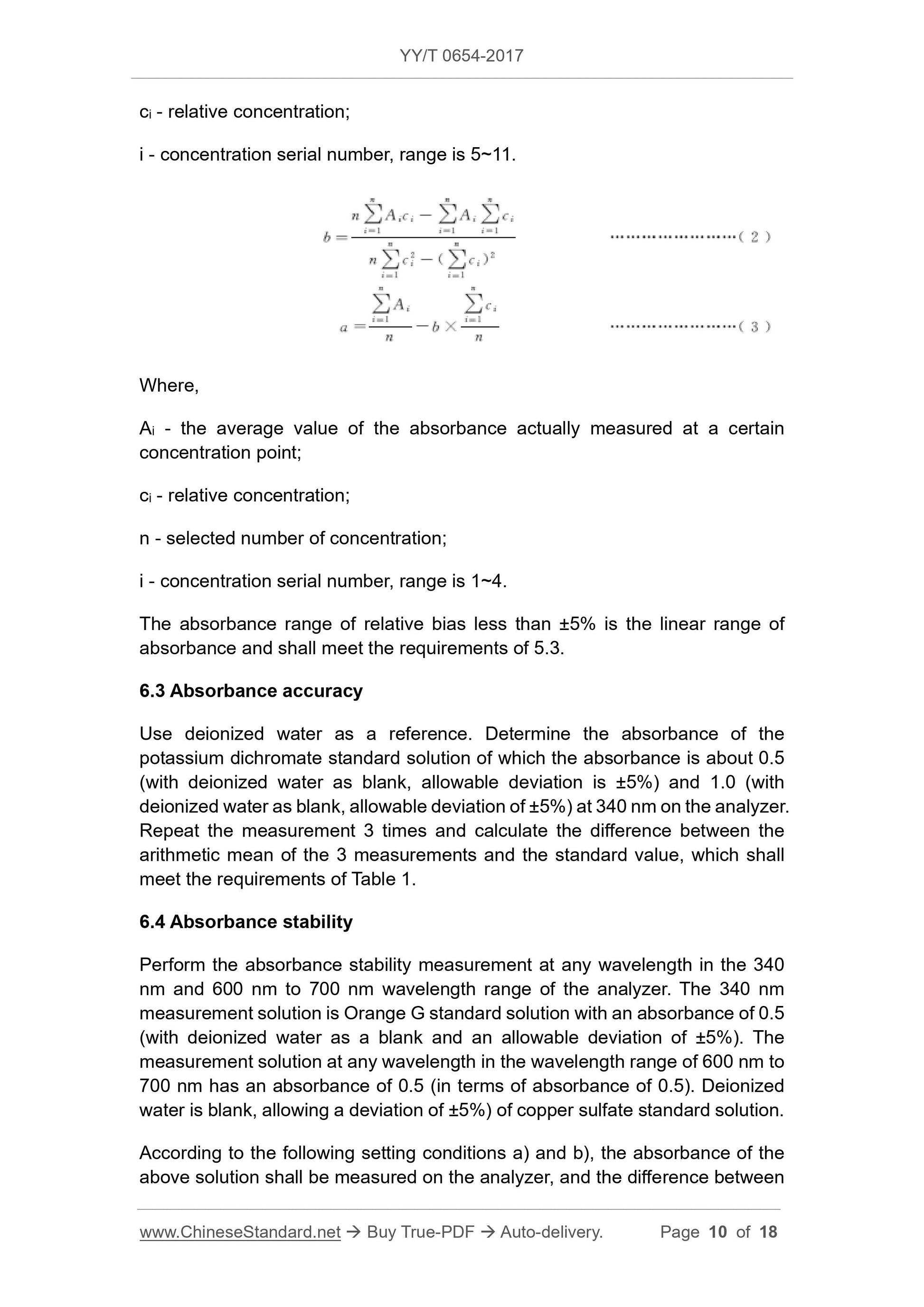
5.3 Absorbance linear range
The maximum absorbance of the relative bias within ±5% shall be no less than
2.0.
5.4 Absorbance accuracy
Comply with Table 1.
Table 1 -- Absorbance accuracy requirements
Absorbance value Allowable error
0.5 ±0.025
1.0 ±0.07
5.5 Absorbance stability
The change in absorbance shall not be greater than 0.01.
5.6 Absorbance repeatability
Expressed by coefficient of variation, not exceeding 1.5%.
5.7 Temperature accuracy and volatility
The temperature value is within ±0.3°C of the set value, and the fluctuation is
not more than ±0.2°C.
5.8 Sample carry-over rate
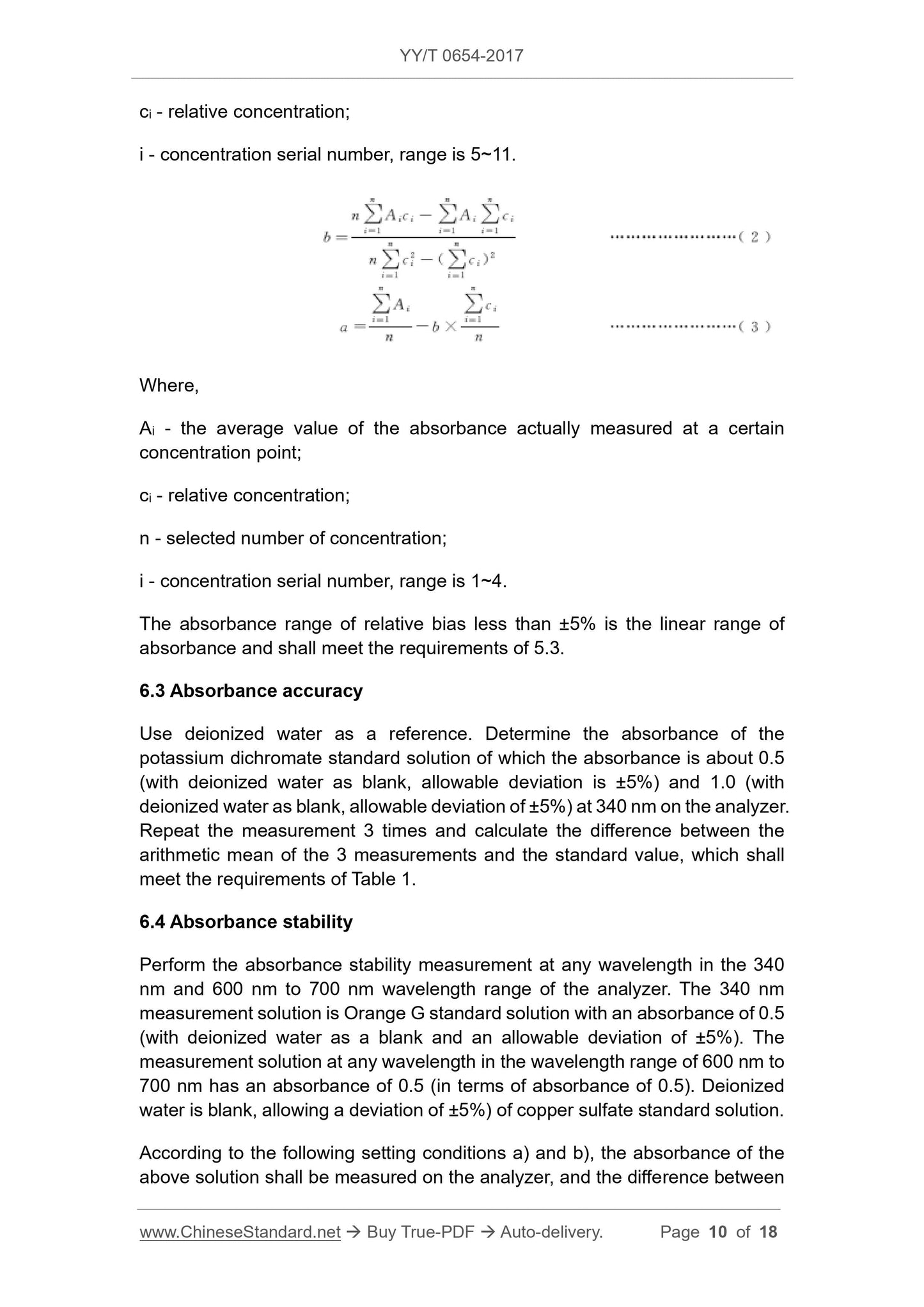
ci - relative concentration;
i - concentration serial number, range is 5~11.
Where,
Ai - the average value of the absorbance actually measured at a certain
concentration point;
ci - relative concentration;
n - selected number of concentration;
i - concentration serial number, range is 1~4.
The absorbance range of relative bias less than ±5% is the linear range of
absorbance and shall meet the requirements of 5.3.
6.3 Absorbance accuracy
Use deionized water as a reference. Determine the absorbance of the
potassium dichromate standard solution of which the absorbance is about 0.5
(with deionized water as blank, allowable deviation is ±5%) and 1.0 (with
deionized water as blank, allowable deviation of ±5%) at 340 nm on the analyzer.
Repeat the measurement 3 times and calculate the difference between the
arithmetic mean of the 3 measurements and the standard value, which shall
meet the requirements of Table 1.
6.4 Absorbance stability
Perform the absorbance stability measurement at any wavelength in the 340
nm and 600 nm to 700 nm wavelength range of the analyzer. The 340 nm
measurement solution is Orange G standard solution with an absorbance of 0.5
(with deionized water as a blank and an allowable deviation of ±5%). The
measurement solution at any wavelength in the wavelength range of 600 nm to
700 nm has an absorbance of 0.5 (in terms of absorbance of 0.5). Deionized
water is blank, allowing a deviation of ±5%) of copper sulfate standard solution.
According to the following setting conditions a) and b), the absorbance of the
above solution shall be measured on the analyzer, and the difference between
Vr - reagent addition volume.
NOTE 1. Surfactants can be added to deionized water (such as TritonX-100).
NOTE 2. Allow adoption of 600nm~700nm as a sub-wavelength.
6.8 Sample loading accuracy and repeatabi...
Share