1
/
dari
10
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
GB 1886.306-2020 English PDF (GB1886.306-2020)
GB 1886.306-2020 English PDF (GB1886.306-2020)
Harga reguler
$110.00 USD
Harga reguler
Harga obral
$110.00 USD
Harga satuan
/
per
Biaya pengiriman dihitung saat checkout.
Tidak dapat memuat ketersediaan pengambilan
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click GB 1886.306-2020
Historical versions: GB 1886.306-2020
Preview True-PDF (Reload/Scroll if blank)
GB 1886.306-2020: National food safety standard - Food additive - Monosodium L-glutamate
GB 1886.306-2020
GB
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National Food Safety Standard -
Food Additive - Monosodium Glutamate
ISSUED ON: SEPTEMBER 11, 2020
IMPLEMENTED ON: MARCH 11, 2021
Issued by: National Health Commission of the PRC;
State Administration for Market Regulation.
Table of Contents
1 Scope ... 3
2 Chemical Name, Structural Formula, Molecular Formula and Relative
Molecular Mass ... 3
3 Technical Requirements ... 3
Appendix A Test Methods ... 5
National Food Safety Standard -
Food Additive - Monosodium Glutamate
1 Scope
This Standard is applicable to food additive of monosodium glutamate made from
carbohydrates (such as starch, corn, molasses and other sugariness) through the
fermentation, extraction, neutralization, crystallization, separating and drying by
microorganisms such as corynebacterium glutamicum.
2 Chemical Name, Structural Formula, Molecular
Formula and Relative Molecular Mass
2.1 Chemical name
L-glutamate monosodium monohydrate (L-α-aminopentanedioic monosodium
monohydrate)
2.2 Structural formula
2.3 Molecular formula
C5H8NNaO4 • H2O
2.4 Relative molecular mass
187.13 (according to 2016 international relative atomic mass)
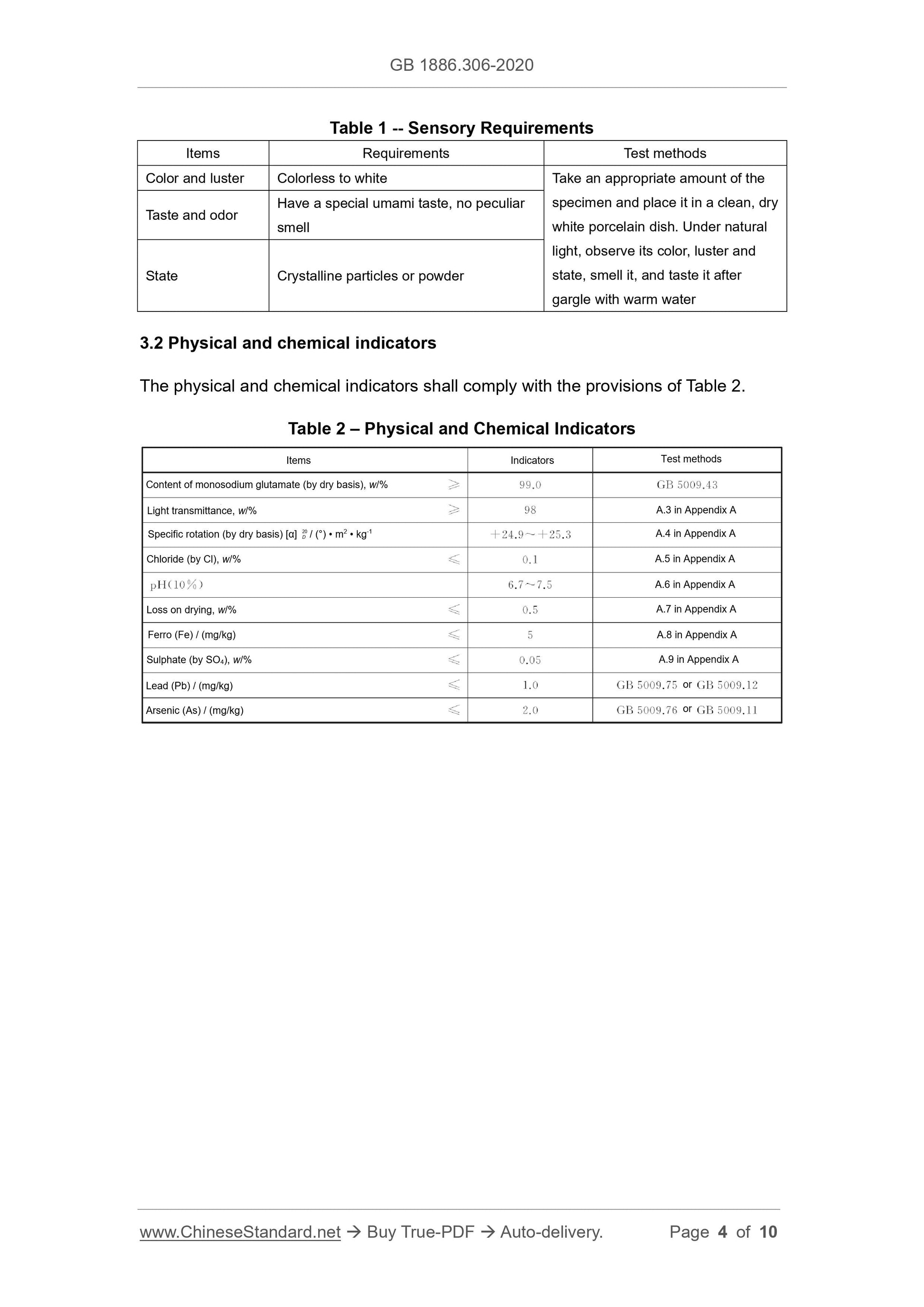
3 Technical Requirements
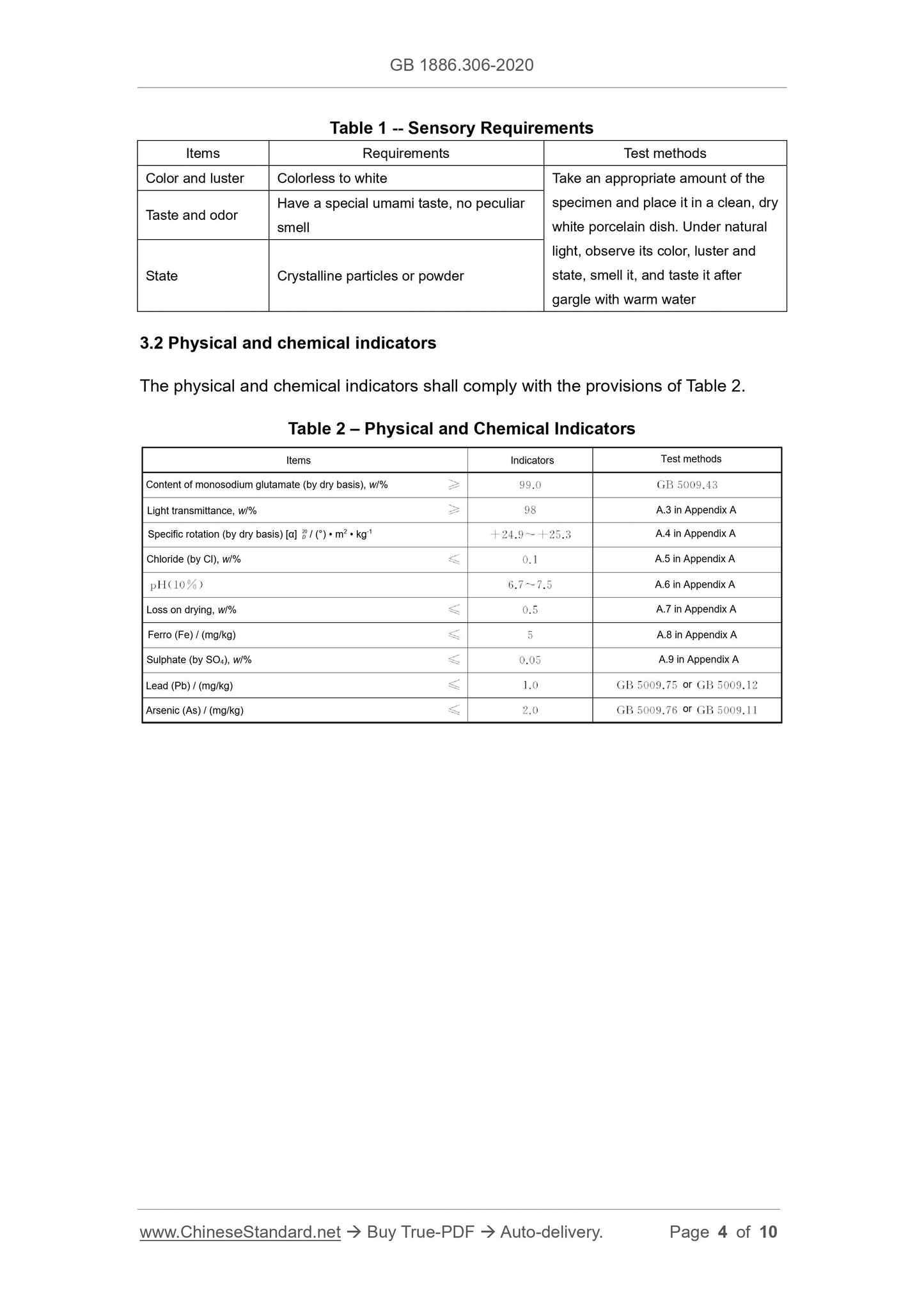
3.1 Sensory requirements
The sensory requirements shall comply with the provisions of Table 1.
Appendix A
Test Methods
A.1 General rules
The reagents and water used in this standard refer to analytically-pure reagents and
Class-III water specified in GB/T 6682 when other requirements are not indicated. The
standard solutions used in the test, standard solutions for impurity determination,
formulations and products are prepared in accordance with the provisions of GB/T 601,
GB/T 602, and GB/T 603 when other requirements are not specified. The solution used
in the test refers to an aqueous solution when it is not specified which solvent is used
for preparation.
A.2 Identification test
A.2.1 Amino acid test
Prepare 1mg/mL specimen solution; take 5.0mL of specimen solution and add 1.0mL
of 1g/L ninhydrin solution; mix well. Heat it in a boiling water bath for 3min; and take it
out. The final specimen solution shall appear purple.
A.2.2 Sodium salt test
Take about 0.5g of the specimen; add 1mL of hydrochloric acid to dissolve it; and insert
the tip of the platinum needle into the test solution about 5mm. After that, put the
platinum needle flat in the colorless or blue flame of the Bunsen burner; and it shall
show a yellow flame and last about 4s.
A.3 Determination of light transmittance
A.3.1 Apparatus
Spectrophotometer: equipped with 1cm cuvette.
A.3.2 Analysis procedures
Take 10g of the specimen, accurate to 0.1g; add water to dissolve; make the constant
volume to 100mL; shake well. Use a 1cm cuvette with water as a blank control;
measure the light transmittance of the specimen solution at a wavelength of 430nm;
and record the reading.
The test results are based on the arithmetic mean of the parallel determination results.
The absolute difference between two independent determination results obtained
under repeatability conditions is no more than 0.2% of the arithmetic mean.
A.5.1 Reagents and materials
A.5.1.1 Nitric acid solution: 1+9.
A.5.1.2 Silver nitrate solution: 17g/L.
A.5.1.3 Chloride standard solution: 0.1mg/mL.
A.5.2 Apparatus
Nessler colorimetric tube.
A.5.3 Analysis procedures
A.5.3.1 Preparation of specimen solution
Take 10.0g of specimen; add water to dissolve and dilute to a constant volume of
100mL; shake well. Pipette 10.00mL of the above solution in a Nessler colorimetric
tube; add 13mL of water; and shake well.
A.5.3.2 Preparation of control solution
Pipette 10.00mL of the chloride standard solution and place it in a Nessler colorimetric
tube; add 13mL of water; and shake well.
A.5.3.3 Determination
Respectively add 1mL of nitric acid solution and 1mL of silver nitrate standard solution
to the specimen solution and the control solution; shake them well. Place them in a
dark place for 5min; and place them on a black background. Observe the resulting
turbidity from the top of the colorimetric tube.
A.5.4 Judgment of results
The turbidity of the specimen solution is no deeper than the control solution; that is,
the chloride content is less than or equal to 0.1%.
A.6 Determination of pH
A.6.1 Reagents and materials
Phosphate standard buffer solution (pH 6.86): take 3.40g of potassium dihydrogen
phosphate (KH2PO4) and 3.55g of disodium hydrogen phosphate (Na2HPO4) that have
been dried at 120°C for 2h; and add carbon-dioxide-free water to dissolve and make
constant volume to 1000mL; shake well.
A.6.2 Apparatus
pH meter (acidity meter) (accuracy ±0.02pH).
m - the mass of the weighing bottle, in g.
The calculation result is retained to one digit after the decimal point.
The test results are based on the arithmetic mean of the parallel determination results.
The absolute difference between two independent determination results obtained
under repeatability conditions is no more than 10% of the arithmetic mean.
A.8 Determination of Ferro (Fe)
A.8.1 Principle of the method
Under acidic conditions, the ferric ions in the specimen solution react with ammonium
thiocyanate; and its color depth is proportional to the concentration of ferric ions, which
can be colorimetrically determined.
A.8.2 Reagents and materials
A.8.2.1 Nitric acid solution: 1+1.
A.8.2.2 Ammonium thiocyanate solution: take 15.0g of ammonium thiocyanate;
dissolve and make constant volume by water to 100mL.
A.8.2.3 Iron standard solution I: 0.1g/L.
A.8.2.4 Iron standard solution II: pipette 10 mL of iron standard solution I and dilute to
100 mL by water.
A.8.3 Apparatus
Colorimetric tube with stopper: 50mL.
A.8.4 Analysis procedures
Take 1g of the specimen in a colorimetric tube, accurate to 0.1g; add 10mL of water to
dissolve; then add 2mL of nitric acid solution; and shake well. Accurately draw 0.5mL
of iron standard solution II in another colorimetric tube; add 9.5mL of water and 2mL
of nitric acid solution; and shake well. Place the above two tubes in a boiling water bath
and boil for 20min at the same time; take them out; cool to room temperature; and add
10.00mL of ammonium thiocyanate solution to each tube; add water to the 25mL mark;
shake well; and perform visual colorimetry.
A.8.5 Judgment of results
The color of the specimen solution is no darker than the standard solution; that is, the
iron content is less than or equal to 5mg/kg.
A.9 Determination of sulfate (by SO4)
Get QUOTATION in 1-minute: Click GB 1886.306-2020
Historical versions: GB 1886.306-2020
Preview True-PDF (Reload/Scroll if blank)
GB 1886.306-2020: National food safety standard - Food additive - Monosodium L-glutamate
GB 1886.306-2020
GB
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National Food Safety Standard -
Food Additive - Monosodium Glutamate
ISSUED ON: SEPTEMBER 11, 2020
IMPLEMENTED ON: MARCH 11, 2021
Issued by: National Health Commission of the PRC;
State Administration for Market Regulation.
Table of Contents
1 Scope ... 3
2 Chemical Name, Structural Formula, Molecular Formula and Relative
Molecular Mass ... 3
3 Technical Requirements ... 3
Appendix A Test Methods ... 5
National Food Safety Standard -
Food Additive - Monosodium Glutamate
1 Scope
This Standard is applicable to food additive of monosodium glutamate made from
carbohydrates (such as starch, corn, molasses and other sugariness) through the
fermentation, extraction, neutralization, crystallization, separating and drying by
microorganisms such as corynebacterium glutamicum.
2 Chemical Name, Structural Formula, Molecular
Formula and Relative Molecular Mass
2.1 Chemical name
L-glutamate monosodium monohydrate (L-α-aminopentanedioic monosodium
monohydrate)
2.2 Structural formula
2.3 Molecular formula
C5H8NNaO4 • H2O
2.4 Relative molecular mass
187.13 (according to 2016 international relative atomic mass)
3 Technical Requirements
3.1 Sensory requirements
The sensory requirements shall comply with the provisions of Table 1.
Appendix A
Test Methods
A.1 General rules
The reagents and water used in this standard refer to analytically-pure reagents and
Class-III water specified in GB/T 6682 when other requirements are not indicated. The
standard solutions used in the test, standard solutions for impurity determination,
formulations and products are prepared in accordance with the provisions of GB/T 601,
GB/T 602, and GB/T 603 when other requirements are not specified. The solution used
in the test refers to an aqueous solution when it is not specified which solvent is used
for preparation.
A.2 Identification test
A.2.1 Amino acid test
Prepare 1mg/mL specimen solution; take 5.0mL of specimen solution and add 1.0mL
of 1g/L ninhydrin solution; mix well. Heat it in a boiling water bath for 3min; and take it
out. The final specimen solution shall appear purple.
A.2.2 Sodium salt test
Take about 0.5g of the specimen; add 1mL of hydrochloric acid to dissolve it; and insert
the tip of the platinum needle into the test solution about 5mm. After that, put the
platinum needle flat in the colorless or blue flame of the Bunsen burner; and it shall
show a yellow flame and last about 4s.
A.3 Determination of light transmittance
A.3.1 Apparatus
Spectrophotometer: equipped with 1cm cuvette.
A.3.2 Analysis procedures
Take 10g of the specimen, accurate to 0.1g; add water to dissolve; make the constant
volume to 100mL; shake well. Use a 1cm cuvette with water as a blank control;
measure the light transmittance of the specimen solution at a wavelength of 430nm;
and record the reading.
The test results are based on the arithmetic mean of the parallel determination results.
The absolute difference between two independent determination results obtained
under repeatability conditions is no more than 0.2% of the arithmetic mean.
A.5.1 Reagents and materials
A.5.1.1 Nitric acid solution: 1+9.
A.5.1.2 Silver nitrate solution: 17g/L.
A.5.1.3 Chloride standard solution: 0.1mg/mL.
A.5.2 Apparatus
Nessler colorimetric tube.
A.5.3 Analysis procedures
A.5.3.1 Preparation of specimen solution
Take 10.0g of specimen; add water to dissolve and dilute to a constant volume of
100mL; shake well. Pipette 10.00mL of the above solution in a Nessler colorimetric
tube; add 13mL of water; and shake well.
A.5.3.2 Preparation of control solution
Pipette 10.00mL of the chloride standard solution and place it in a Nessler colorimetric
tube; add 13mL of water; and shake well.
A.5.3.3 Determination
Respectively add 1mL of nitric acid solution and 1mL of silver nitrate standard solution
to the specimen solution and the control solution; shake them well. Place them in a
dark place for 5min; and place them on a black background. Observe the resulting
turbidity from the top of the colorimetric tube.
A.5.4 Judgment of results
The turbidity of the specimen solution is no deeper than the control solution; that is,
the chloride content is less than or equal to 0.1%.
A.6 Determination of pH
A.6.1 Reagents and materials
Phosphate standard buffer solution (pH 6.86): take 3.40g of potassium dihydrogen
phosphate (KH2PO4) and 3.55g of disodium hydrogen phosphate (Na2HPO4) that have
been dried at 120°C for 2h; and add carbon-dioxide-free water to dissolve and make
constant volume to 1000mL; shake well.
A.6.2 Apparatus
pH meter (acidity meter) (accuracy ±0.02pH).
m - the mass of the weighing bottle, in g.
The calculation result is retained to one digit after the decimal point.
The test results are based on the arithmetic mean of the parallel determination results.
The absolute difference between two independent determination results obtained
under repeatability conditions is no more than 10% of the arithmetic mean.
A.8 Determination of Ferro (Fe)
A.8.1 Principle of the method
Under acidic conditions, the ferric ions in the specimen solution react with ammonium
thiocyanate; and its color depth is proportional to the concentration of ferric ions, which
can be colorimetrically determined.
A.8.2 Reagents and materials
A.8.2.1 Nitric acid solution: 1+1.
A.8.2.2 Ammonium thiocyanate solution: take 15.0g of ammonium thiocyanate;
dissolve and make constant volume by water to 100mL.
A.8.2.3 Iron standard solution I: 0.1g/L.
A.8.2.4 Iron standard solution II: pipette 10 mL of iron standard solution I and dilute to
100 mL by water.
A.8.3 Apparatus
Colorimetric tube with stopper: 50mL.
A.8.4 Analysis procedures
Take 1g of the specimen in a colorimetric tube, accurate to 0.1g; add 10mL of water to
dissolve; then add 2mL of nitric acid solution; and shake well. Accurately draw 0.5mL
of iron standard solution II in another colorimetric tube; add 9.5mL of water and 2mL
of nitric acid solution; and shake well. Place the above two tubes in a boiling water bath
and boil for 20min at the same time; take them out; cool to room temperature; and add
10.00mL of ammonium thiocyanate solution to each tube; add water to the 25mL mark;
shake well; and perform visual colorimetry.
A.8.5 Judgment of results
The color of the specimen solution is no darker than the standard solution; that is, the
iron content is less than or equal to 5mg/kg.
A.9 Determination of sulfate (by SO4)
Share