1
/
su
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
GB 1886.15-2015 English PDF (GB1886.15-2015)
GB 1886.15-2015 English PDF (GB1886.15-2015)
Prezzo di listino
$140.00 USD
Prezzo di listino
Prezzo scontato
$140.00 USD
Prezzo unitario
/
per
Spese di spedizione calcolate al check-out.
Impossibile caricare la disponibilità di ritiro
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click GB 1886.15-2015
Historical versions: GB 1886.15-2015
Preview True-PDF (Reload/Scroll if blank)
GB 1886.15-2015: National Food Safety Standard -- Food Additives -- Phosphoric acid
GB 1886.15-2015
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National Food Safety Standard
- Food Additives - Phosphoric Acid
ISSUED ON: SEPTEMBER 22, 2015
IMPLEMENTED ON: MARCH 22, 2016
Issued by: National Health and Family Planning Commission of PRC
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Molecular Formula and Relative Molecular Mass ... 4
3 Technical Requirements ... 4
Appendix A Test Methods ... 6
National Food Safety Standard
– Food Additives – Phosphoric Acid
1 Scope
This Standard is applicable to food additive of phosphoric acid produced by thermal
method.
2 Molecular Formula and Relative Molecular Mass
2.1 Molecular formula
H3PO4
2.2 Relative molecular mass
97.99 (according to international relative atomic mass in 2007)
3 Technical Requirements
3.1 Sensory requirements
The sensory requirements shall meet the requirements of Table 1.
3.2 Physical and chemical indicators
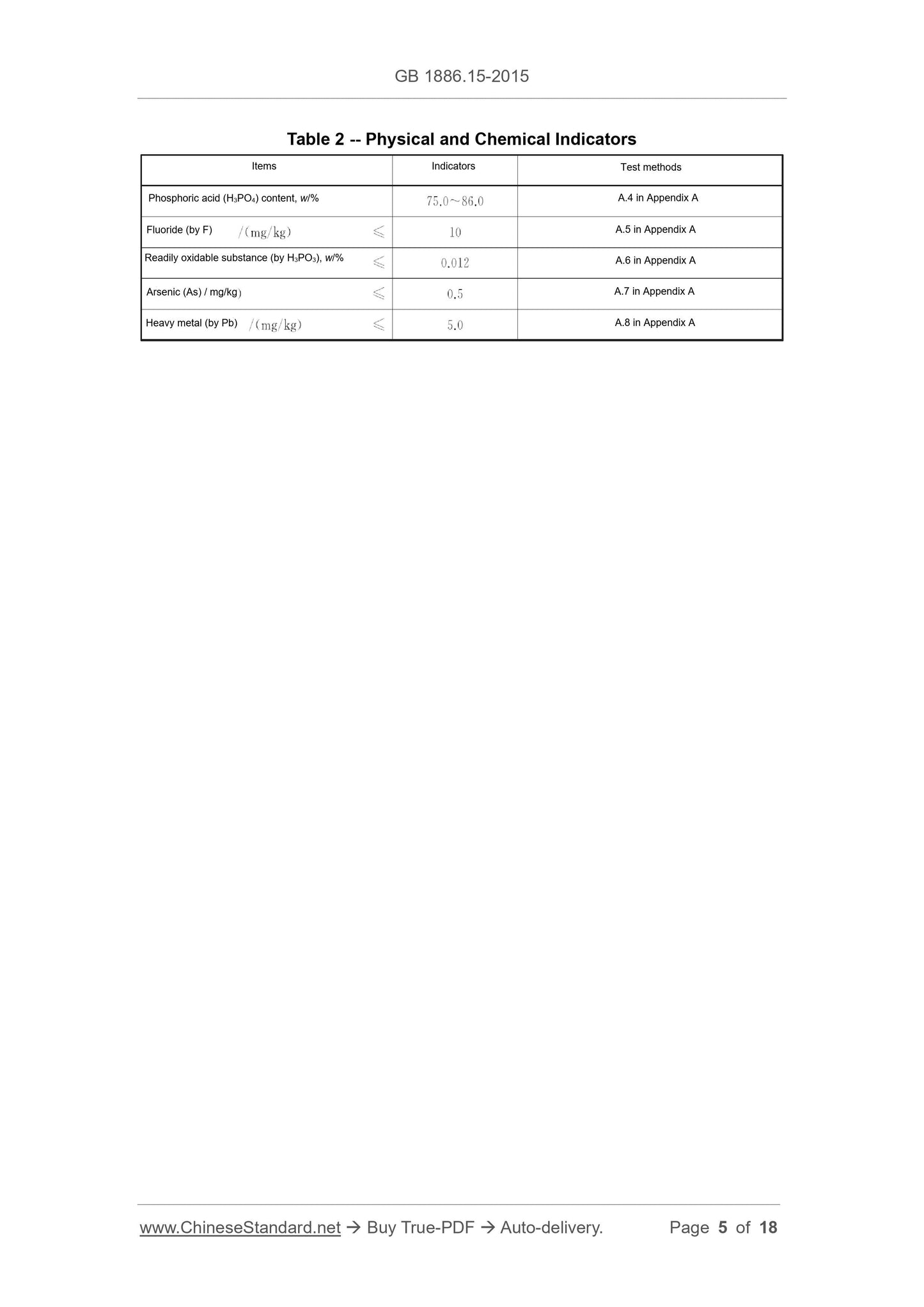
The physical and chemical indicators shall conform to the provisions of Table 2.
weighing, the phosphoric acid content was determined.
A.4.1.2 Reagents and materials
A.4.1.2.1 Hydrochloric acid.
A.4.1.2.2 Preparation of quimociac solution
a) Take 70g of sodium molybdate to dissolve in 150mL of water; this solution is
Solution-A;
b) Take 60g of citric acid to dissolve in a mixed solution of 150mL of water and 85mL
of nitric acid; this solution is Solution-B;
c) Pour Solution-A into Solution-B under stirring condition; this solution is Solution-
C;
d) Add 35 mL of nitric acid to 100 mL of water, and then add 5 mL of quinoline; this
solution is Solution-D;
e) Pour Solution-D into Solution-C and mix well. After standing for 12h, filter by a
glass sand crucible; add 280 mL of acetone; dilute with water to 1000 mL and
mix well; and store in a polyethylene bottle.
A.4.1.3 instruments and equipment
A.4.1.3.1 Glass sand crucible: filter plate pore size 5μm~15μm.
A.4.1.3.2 Electric oven: the temperature can be controlled at 180°C±5°C or
250°C±10°C.
A.4.1.4 Analysis procedures
A.4.1.4.1 Preparation of test solution
Take about 1g of specimen, accurate to 0.0002g; put it in a 100mL beaker; add 5mL of
hydrochloric acid and an appropriate amount of water; cover the watch glass; boil for
10min; after cooling, transfer to a 500mL volumetric flask; add 10mL of hydrochloric
acid, and dilute with water to the mark, shake well.
A.4.1.4.2 Preparation of blank test solution
While preparing the test solution, except for not adding specimen, other operations
and the added amounts of reagents are the same as those in A.4.1.4.1.
A.4.1.4.3 Determination
Pipette 10mL of test solution and blank test solution into 250mL beakers respectively;
A.5.2 Reagents and materials
A.5.2.1 Hydrochloric acid solution: 1+1.
A.5.2.2 Nitric acid solution: 1+15.
A.5.2.3 Sodium hydroxide solution: 200 g/L.
A.5.2.4 Citric acid - trisodium citrate buffer solution: pH = 5.5~6. Take 270g of trisodium
citrate (Na3C6H5O7 · 2H2O) and 24g of citric acid (C6H6O7 · H2O) to dissolve in water,
dilute to 1000mL, and mix well.
A.5.2.5 Bromocresol green indicator liquid: 1 g/L.
A.5.2.6 Fluoride standard solution: 1mL of such solution contains 2μg of fluorine (F).
Prepare just before use. Pipette 2mL of the fluoride standard solution prepared
according to GB/T 602; place it in a 100mL volumetric flask; dilute with water to the
mark; and shake well.
A.5.3 Instruments and equipment
A.5.3.1 Fluoride ion selective electrode.
A.5.3.2 Saturated calomel electrode.
A.5.3.3 Potentiometer: The accuracy is 2mV/div.
A.5.3.4 Electromagnetic stirrer.
A.5.4 Analysis procedures
A.5.4.1 Drawing of working curve
Pipette 0.00mL, 1.00mL, 3.00mL, 5.00mL, 7.00mL, 10.00mL of fluoride standard
solutions into the 50mL volumetric flasks, respectively; add 1mL hydrochloric acid
solution, 5 drops of citric acid - trisodium citrate buffer solution and 2 drops of
bromocresol green indicator liquid; adjust the solution to blue by sodium hydroxide
solution; then adjust the solution to just yellow by nitric acid solution; add 20mL of citric
acid - trisodium citrate buffer solution; and dilute with water to scale and mix well. Pour
the solution into a 50mL dry beaker; place it on an electromagnetic stirrer; insert a
fluoride ion selective electrode and a saturated calomel electrode; connect the
potentiometer; stir for a while; adjust the potentiometer to zero point; then measure;
and record the potential at equilibrium value. Draw the standard curve with the
logarithmic value of the value of the mass of fluoride (by F) as the abscissa and the
corresponding potential value as the ordinate.
A.5.4.2 Preparation of test solution
In cerium sulfate standard titration solution with excessive acid, it reacts with the
readable oxidizable substance in the specimen. Titrate the excessive cerium sulfate
standard titration solution by ammonium ferrous sulfate standard titration solution.
A.6.2 Reagents and materials
A.6.2.1 Sulfuric acid solution: 1+2.
A.6.2.2 Silver sulfate solution (10 g/L): take 1g of silver sulfate; add 50mL of sulfuric
acid solution (1+2) to dissolve; dilute with water to 100mL; and shake well.
A.6.2.3 Cerium sulfate standard titration solution: c[Ce(SO4) 2] = 0.1 mol/L.
A.6.2.4 Ammonium ferrous sulfate Standard titration solution: c[(NH4)2Fe(SO4)2] = 0.1
mol/L.
A.6.2.5 1,10-phenanthroline-ferrous iron indicator liquid.
A.6.3 Analysis procedures
In a 250mL conical flask, add 40mL of sulfuric acid solution; use a pipette to transfer
10.00mL of cerium sulfate standard titration solution; and then add 10mL of silver
sulfate solution; shake well. Add 10g (about 6mL) of the specimen dropwise; add 40
mL of water; boil for 15 min, and cool. Dilute with water to the original volume; add 2
drops of 1,10-phenanthroline-ferrous iron indicator liquid; and titrate by ammonium
ferrous sulfate standard titration solution until the solution turns red. Do a blank test at
the same time.
A.6.4 Calculation of results
Mass fraction, w3, of readily oxidizable substance (by H3PO3) is calculated according
to Formula (A.4):
Where:
V1 – volume of ammonium ferrous sulfate standard titration solution (A.6.2.4)
consumed in the blank test, in mL;
V2 – volume of ammonium ferrous sulfate standard titration solution (A.6.2.4)
consumed during the titration, in mL;
1000 – conversion coefficient between mL and L;
corresponding absorbance as the ordinate to draw a working curve.
A.7.1.4.2 Determination
Take about 10g of specimen, accurate to 0.01g; put it in the arsenic generation bottle;
add water to 40mL; and mix well. Add 20mL of sulfuric acid solution and mix well. Add
2mL of potassium iodide solution and 2mL of stannous chloride solution; and shake
well. Stand for 15min. Add 3mL of silver diethyldithiocarbamate-pyridine solution to the
absorption tube containing lead acetate cotton; add 3g of arsenic-free metal zinc
particles to the arsenic generation bottle; and immediately connect the absorption tube
to the arsenic generation bottle. After the reaction is complete (approximately 45min at
normal temperature), the absorbance of the absorption solution in the absorption tube
is measured at 540nm by a 1cm colorimetric cell. Subtract the absorbance of the blank
solution from the absorbance of the test solution, and find the corresponding arsenic
(As) content from the working curve.
A.7.1.5 Calculation of results
Mass fraction, w4,...
Get QUOTATION in 1-minute: Click GB 1886.15-2015
Historical versions: GB 1886.15-2015
Preview True-PDF (Reload/Scroll if blank)
GB 1886.15-2015: National Food Safety Standard -- Food Additives -- Phosphoric acid
GB 1886.15-2015
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National Food Safety Standard
- Food Additives - Phosphoric Acid
ISSUED ON: SEPTEMBER 22, 2015
IMPLEMENTED ON: MARCH 22, 2016
Issued by: National Health and Family Planning Commission of PRC
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Molecular Formula and Relative Molecular Mass ... 4
3 Technical Requirements ... 4
Appendix A Test Methods ... 6
National Food Safety Standard
– Food Additives – Phosphoric Acid
1 Scope
This Standard is applicable to food additive of phosphoric acid produced by thermal
method.
2 Molecular Formula and Relative Molecular Mass
2.1 Molecular formula
H3PO4
2.2 Relative molecular mass
97.99 (according to international relative atomic mass in 2007)
3 Technical Requirements
3.1 Sensory requirements
The sensory requirements shall meet the requirements of Table 1.
3.2 Physical and chemical indicators
The physical and chemical indicators shall conform to the provisions of Table 2.
weighing, the phosphoric acid content was determined.
A.4.1.2 Reagents and materials
A.4.1.2.1 Hydrochloric acid.
A.4.1.2.2 Preparation of quimociac solution
a) Take 70g of sodium molybdate to dissolve in 150mL of water; this solution is
Solution-A;
b) Take 60g of citric acid to dissolve in a mixed solution of 150mL of water and 85mL
of nitric acid; this solution is Solution-B;
c) Pour Solution-A into Solution-B under stirring condition; this solution is Solution-
C;
d) Add 35 mL of nitric acid to 100 mL of water, and then add 5 mL of quinoline; this
solution is Solution-D;
e) Pour Solution-D into Solution-C and mix well. After standing for 12h, filter by a
glass sand crucible; add 280 mL of acetone; dilute with water to 1000 mL and
mix well; and store in a polyethylene bottle.
A.4.1.3 instruments and equipment
A.4.1.3.1 Glass sand crucible: filter plate pore size 5μm~15μm.
A.4.1.3.2 Electric oven: the temperature can be controlled at 180°C±5°C or
250°C±10°C.
A.4.1.4 Analysis procedures
A.4.1.4.1 Preparation of test solution
Take about 1g of specimen, accurate to 0.0002g; put it in a 100mL beaker; add 5mL of
hydrochloric acid and an appropriate amount of water; cover the watch glass; boil for
10min; after cooling, transfer to a 500mL volumetric flask; add 10mL of hydrochloric
acid, and dilute with water to the mark, shake well.
A.4.1.4.2 Preparation of blank test solution
While preparing the test solution, except for not adding specimen, other operations
and the added amounts of reagents are the same as those in A.4.1.4.1.
A.4.1.4.3 Determination
Pipette 10mL of test solution and blank test solution into 250mL beakers respectively;
A.5.2 Reagents and materials
A.5.2.1 Hydrochloric acid solution: 1+1.
A.5.2.2 Nitric acid solution: 1+15.
A.5.2.3 Sodium hydroxide solution: 200 g/L.
A.5.2.4 Citric acid - trisodium citrate buffer solution: pH = 5.5~6. Take 270g of trisodium
citrate (Na3C6H5O7 · 2H2O) and 24g of citric acid (C6H6O7 · H2O) to dissolve in water,
dilute to 1000mL, and mix well.
A.5.2.5 Bromocresol green indicator liquid: 1 g/L.
A.5.2.6 Fluoride standard solution: 1mL of such solution contains 2μg of fluorine (F).
Prepare just before use. Pipette 2mL of the fluoride standard solution prepared
according to GB/T 602; place it in a 100mL volumetric flask; dilute with water to the
mark; and shake well.
A.5.3 Instruments and equipment
A.5.3.1 Fluoride ion selective electrode.
A.5.3.2 Saturated calomel electrode.
A.5.3.3 Potentiometer: The accuracy is 2mV/div.
A.5.3.4 Electromagnetic stirrer.
A.5.4 Analysis procedures
A.5.4.1 Drawing of working curve
Pipette 0.00mL, 1.00mL, 3.00mL, 5.00mL, 7.00mL, 10.00mL of fluoride standard
solutions into the 50mL volumetric flasks, respectively; add 1mL hydrochloric acid
solution, 5 drops of citric acid - trisodium citrate buffer solution and 2 drops of
bromocresol green indicator liquid; adjust the solution to blue by sodium hydroxide
solution; then adjust the solution to just yellow by nitric acid solution; add 20mL of citric
acid - trisodium citrate buffer solution; and dilute with water to scale and mix well. Pour
the solution into a 50mL dry beaker; place it on an electromagnetic stirrer; insert a
fluoride ion selective electrode and a saturated calomel electrode; connect the
potentiometer; stir for a while; adjust the potentiometer to zero point; then measure;
and record the potential at equilibrium value. Draw the standard curve with the
logarithmic value of the value of the mass of fluoride (by F) as the abscissa and the
corresponding potential value as the ordinate.
A.5.4.2 Preparation of test solution
In cerium sulfate standard titration solution with excessive acid, it reacts with the
readable oxidizable substance in the specimen. Titrate the excessive cerium sulfate
standard titration solution by ammonium ferrous sulfate standard titration solution.
A.6.2 Reagents and materials
A.6.2.1 Sulfuric acid solution: 1+2.
A.6.2.2 Silver sulfate solution (10 g/L): take 1g of silver sulfate; add 50mL of sulfuric
acid solution (1+2) to dissolve; dilute with water to 100mL; and shake well.
A.6.2.3 Cerium sulfate standard titration solution: c[Ce(SO4) 2] = 0.1 mol/L.
A.6.2.4 Ammonium ferrous sulfate Standard titration solution: c[(NH4)2Fe(SO4)2] = 0.1
mol/L.
A.6.2.5 1,10-phenanthroline-ferrous iron indicator liquid.
A.6.3 Analysis procedures
In a 250mL conical flask, add 40mL of sulfuric acid solution; use a pipette to transfer
10.00mL of cerium sulfate standard titration solution; and then add 10mL of silver
sulfate solution; shake well. Add 10g (about 6mL) of the specimen dropwise; add 40
mL of water; boil for 15 min, and cool. Dilute with water to the original volume; add 2
drops of 1,10-phenanthroline-ferrous iron indicator liquid; and titrate by ammonium
ferrous sulfate standard titration solution until the solution turns red. Do a blank test at
the same time.
A.6.4 Calculation of results
Mass fraction, w3, of readily oxidizable substance (by H3PO3) is calculated according
to Formula (A.4):
Where:
V1 – volume of ammonium ferrous sulfate standard titration solution (A.6.2.4)
consumed in the blank test, in mL;
V2 – volume of ammonium ferrous sulfate standard titration solution (A.6.2.4)
consumed during the titration, in mL;
1000 – conversion coefficient between mL and L;
corresponding absorbance as the ordinate to draw a working curve.
A.7.1.4.2 Determination
Take about 10g of specimen, accurate to 0.01g; put it in the arsenic generation bottle;
add water to 40mL; and mix well. Add 20mL of sulfuric acid solution and mix well. Add
2mL of potassium iodide solution and 2mL of stannous chloride solution; and shake
well. Stand for 15min. Add 3mL of silver diethyldithiocarbamate-pyridine solution to the
absorption tube containing lead acetate cotton; add 3g of arsenic-free metal zinc
particles to the arsenic generation bottle; and immediately connect the absorption tube
to the arsenic generation bottle. After the reaction is complete (approximately 45min at
normal temperature), the absorbance of the absorption solution in the absorption tube
is measured at 540nm by a 1cm colorimetric cell. Subtract the absorbance of the blank
solution from the absorbance of the test solution, and find the corresponding arsenic
(As) content from the working curve.
A.7.1.5 Calculation of results
Mass fraction, w4,...
Share