1
/
su
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 0043-2016 English PDF (YYT0043-2016)
YY/T 0043-2016 English PDF (YYT0043-2016)
Prezzo di listino
$260.00 USD
Prezzo di listino
Prezzo scontato
$260.00 USD
Prezzo unitario
/
per
Spese di spedizione calcolate al check-out.
Impossibile caricare la disponibilità di ritiro
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY/T 0043-2016
Historical versions: YY/T 0043-2016
Preview True-PDF (Reload/Scroll if blank)
YY/T 0043-2016: Medical suture needle
YY/T 0043-2016
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.30
C 31
Replacing YY 0043-2005, YY 0666-2008
Medical suture needle
ISSUED ON: MARCH 23, 2016
IMPLEMENTED ON: JANUARY 01, 2017
Issued by: China Food and Drug Administration
Table of Contents
Foreword ... 3
1 Scope ... 5
2 Normative references ... 5
3 Classification, labeling and materials ... 6
4 Requirements ... 7
5 Test method ... 10
6 Inspection rules ... 13
7 Packaging, marking and instructions for use... 13
8 Storage ... 14
Appendix A (Informative) Schematic diagram of suture needle’s form, arc, hole
... 15
Appendix B (Informative) Schematic diagram of test device for elasticity and
toughness of suture needle ... 16
Appendix C (Normative) Test method for piercing force and strength of needle
tip ... 17
Appendix D (Normative) Test method for cutting force of suture needle’s triangle
edge ... 21
Medical suture needle
1 Scope
This standard specifies the product classification, labeling and materials,
requirements, test methods, inspection rules, packaging, markings and
instructions for use and storage of medical suture needles.
This standard applies to medical suture needles (hereinafter referred to as
suture needles) used to suture internal organs, soft tissues, skin, etc.
2 Normative references
The following documents are essential to the application of this document. For
the dated documents, only the versions with the dates indicated are applicable
to this document; for the undated documents, only the latest version (including
all the amendments) are applicable to this standard.
GB/T 4240 Stainless steel wires
GB/T 4340.1 Metallic materials - Vickers hardness test - Part 1: Test method
GB/T 16886.5-2003 Biological evaluation of medical devices - Part 5: Test
for in vitro cytotoxicity
GB/T 16886.7-2001 Biological evaluation of medical devices - Part 7:
Ethylene oxide sterilization residuals
GB/T 16886.10-2005 Biological evaluation of medical devices - Part 10:
Tests for irritation and delayed-type hypersensitivity
YY/T 0149-2006 Medical instruments of stainless steel - Test methods of
corrosion resistance
YY 0167-2005 Non-absorbable surgical suture
YY/T 0171-2008 Surgical instruments - Packaging, marking and instructions
Pharmacopoeia of the People's Republic of China (2010 Edition · Part 2)
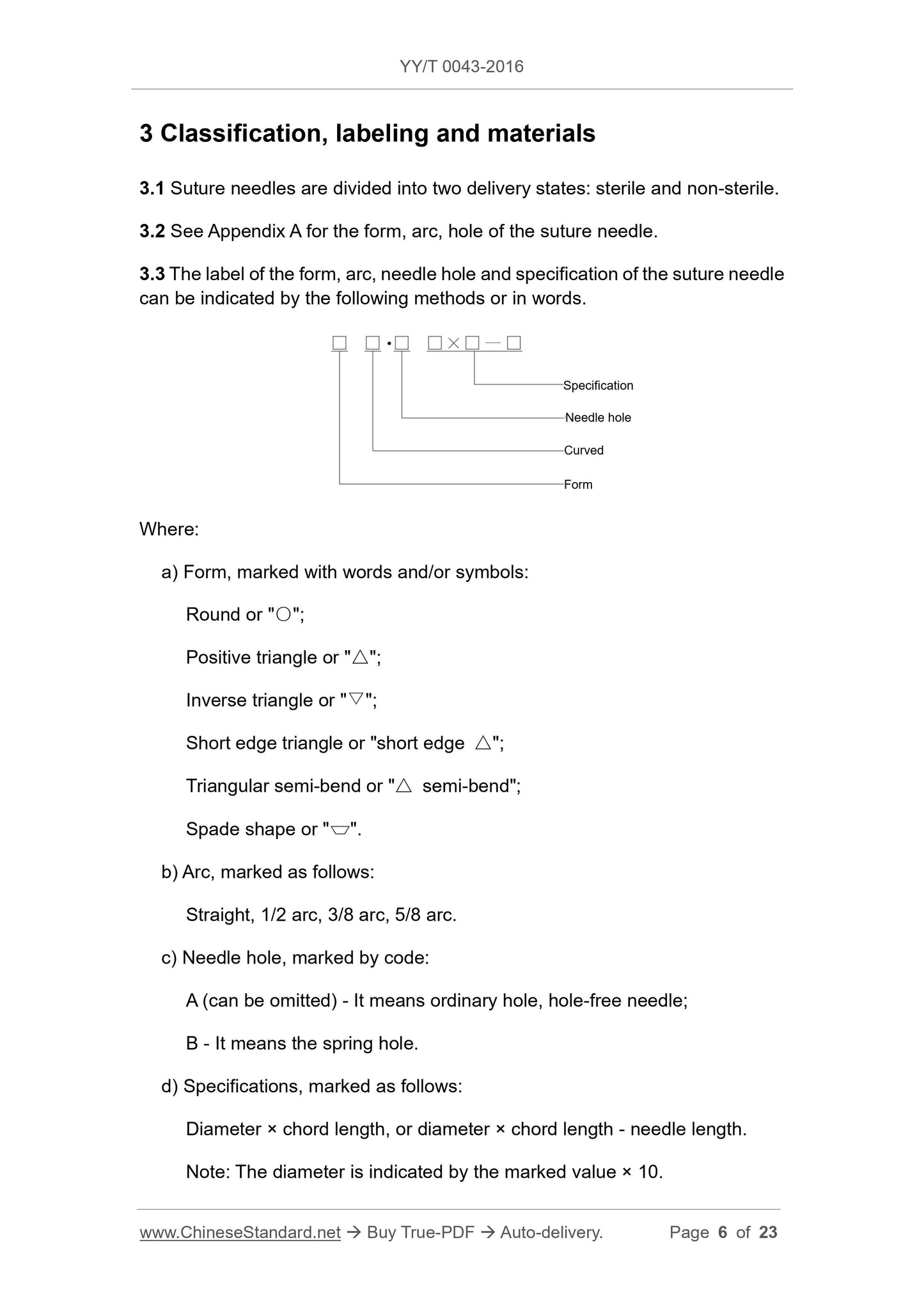
Example:
Diameter D = 0.7 mm, chord length L = 24 mm, needle length = 31 mm, the
needle hole is a common hole, the form is a round needle, the arc is 3/8 arc,
which is marked as:
Round 3/8 arc • 7 × 24, or, round 3/8 arc • 7 × 24 - 31;
○3/8 arc·7 x 24, or, ○3/8 arc·7 x 24 - 31.
3.4 Materials
The suture needle is made of 30Cr13, 40Cr13, 12Cr18Ni9, 06Cr19Ni10
materials as specified in GB/T 4240, or other materials that meet the
requirements of the standard.
4 Requirements
4.1 Appearance
4.1.1 Needle body
The surface of the suture needle shall be smooth and uniform in color; the
needle tip shall have no hooks; the needle hole and needle groove shall be
smooth and centered; there shall be no burrs or obvious skew.
After the suture needle is connected to the suture thread, the needle tail shall
be smooth and free of burr.
4.1.2 Triangular cutting edge
The cutting edge of the triangular needle shall not be curled, white-edged or
jagged.
4.1.3 Surface roughness
The value of surface roughness Rα shall not be greater than 0.8 μm.
4.1.4 Embedding thread of spring hole
There shall be no defects such as secant (broken strand) or off-line in the
embedding thread of spring hole.
4.2 Physical properties
4.2.1 Hardness
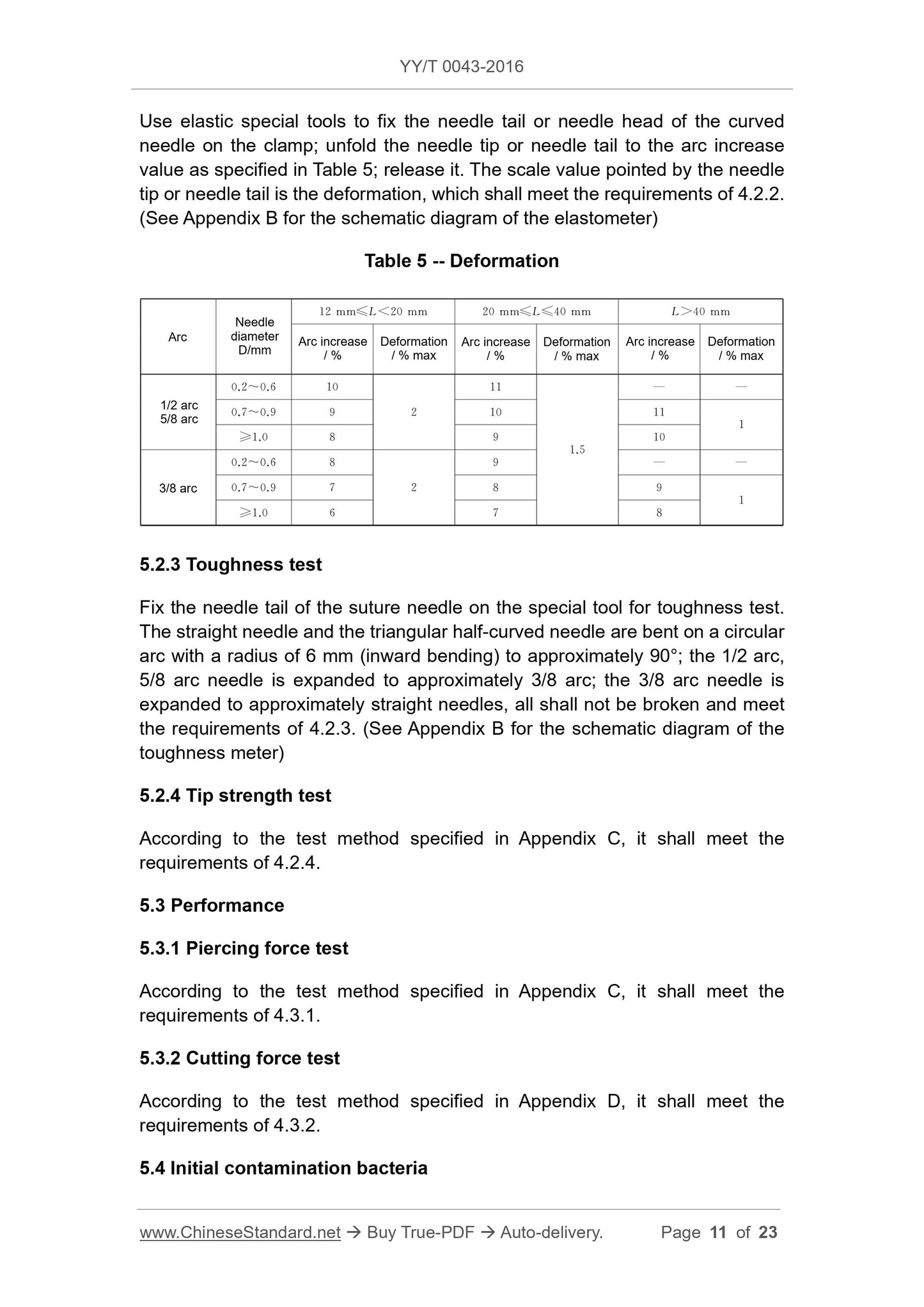
The hardness of the suture needle shall meet the requirements of Table 1.
Carry out testing according to the Appendix XI J Testing Method of Microbial
Limit of the People's Republic of China Pharmacopoeia" (2010 Edition · Part 2),
the results shall comply with the provisions of 4.4.
5.5 Corrosion resistance
The corrosion resistance of the suture needle is tested according to the boiling
water test method of YY/T 0149-2006, the results shall meet the requirements
of 4.5.
5.6 Sterility
Carry out testing according to the Appendix XI H Sterile Test Method of the
People's Republic of China Pharmacopoeia" (2010 Edition · Part 2), the results
shall comply with the provisions of 4.6.
5.7 Residual ethylene oxide
The determination of the residual ethylene oxide is carried out according to the
method specified in GB/T 16886.7-2001, the results shall meet the
requirements of 4.7.
The preparation of the test solution: the suture needle and test water are
prepared in a ratio of 1.0 g:5 mL, sealed and equilibrated at 60 °C ± 1 °C for 40
min.
5.8 Biological evaluation
5.8.1 Carry out according to the method specified in 8.2 (extract liquid test) in
GB/T 16886.5-2003; the results shall meet the requirements of 4.8.1.
Preparation of extract:
a) Extraction conditions (37 ± 1) °C, (24 ± 2) h;
b) Extraction ratio 0.2 g/mL;
c) The extraction medium uses 10% serum.
5.8.2 Carry out according to the method specified in 7.4 (maximum dose test)
in GB/T 16886.10-2005; the results shall meet the requirements of 4.8.2.
5.8.3 Carry out according to the method specified in Appendix B.2 (intradermal
reaction test) in GB/T 16886.10-2005; the results shall meet the requirements
of 4.8.3.
5.9 Packaging and marking
Appendix C
(Normative)
Test method for piercing force and strength of needle tip
C.1 Scope
This method is suitable for testing the needle tip’s piercing force and strength
of suture needles.
C.2 Principle
C.2.1 Evaluate the strength of the suture needle tip by pressing the suture
needle tip vertically against the test material in the specified time under
specified load.
C.2.2 Evaluate the needle tip’s piercing force of the suture needle by the
maximum peak force measured by vertically piercing the test material with the
suture needle at the specified speed.
C.3 Definition
C.3.1
Strength of needles tips
After the needle tip is subject to external force, the force at which hook does
not appear.
C.3.2
Penetration force of needles tips
The force of needle piercing.
C.4 Test equipment
C.4.1 Instrument structure
The instrument structure is as shown in Figure C.1; other instruments with the
same performance and accuracy can also be used (such as adding a
measuring amplifier, data processing and display unit, printer, floppy disk and
other devices).
C.4.2.2 The indication error of the instrument shall not be greater than 0.01 N.
C.4.2.3 The instrument shall be equipped with a level correction and shock-
proof device. The position where the suture needle tips are clamped shall be
adjustable and stable when used.
C.4.2.4 The transmission device of the instrument shall be sensitive and reliable.
When the aluminum foil is pierced in contact with the electrode, the pointer shall
stop automatically.
C.4.2.5 The initial inductance of the instrument shall not be greater than 0.02
N.
C.4.2.6 The aperture of the aluminum foil fixture of the instrument is Φ5 mm;
the aluminum foil after clamping must not be loose; the aluminum foil in the
aperture shall be flat.
C.5 Test materials
C.5.1 Steel block
C.5.1.1 The hardness of the steel block is not less than FRC60.
C.5.1.2 The steel block is a cylinder with a smooth surface and a diamet...
Get QUOTATION in 1-minute: Click YY/T 0043-2016
Historical versions: YY/T 0043-2016
Preview True-PDF (Reload/Scroll if blank)
YY/T 0043-2016: Medical suture needle
YY/T 0043-2016
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.30
C 31
Replacing YY 0043-2005, YY 0666-2008
Medical suture needle
ISSUED ON: MARCH 23, 2016
IMPLEMENTED ON: JANUARY 01, 2017
Issued by: China Food and Drug Administration
Table of Contents
Foreword ... 3
1 Scope ... 5
2 Normative references ... 5
3 Classification, labeling and materials ... 6
4 Requirements ... 7
5 Test method ... 10
6 Inspection rules ... 13
7 Packaging, marking and instructions for use... 13
8 Storage ... 14
Appendix A (Informative) Schematic diagram of suture needle’s form, arc, hole
... 15
Appendix B (Informative) Schematic diagram of test device for elasticity and
toughness of suture needle ... 16
Appendix C (Normative) Test method for piercing force and strength of needle
tip ... 17
Appendix D (Normative) Test method for cutting force of suture needle’s triangle
edge ... 21
Medical suture needle
1 Scope
This standard specifies the product classification, labeling and materials,
requirements, test methods, inspection rules, packaging, markings and
instructions for use and storage of medical suture needles.
This standard applies to medical suture needles (hereinafter referred to as
suture needles) used to suture internal organs, soft tissues, skin, etc.
2 Normative references
The following documents are essential to the application of this document. For
the dated documents, only the versions with the dates indicated are applicable
to this document; for the undated documents, only the latest version (including
all the amendments) are applicable to this standard.
GB/T 4240 Stainless steel wires
GB/T 4340.1 Metallic materials - Vickers hardness test - Part 1: Test method
GB/T 16886.5-2003 Biological evaluation of medical devices - Part 5: Test
for in vitro cytotoxicity
GB/T 16886.7-2001 Biological evaluation of medical devices - Part 7:
Ethylene oxide sterilization residuals
GB/T 16886.10-2005 Biological evaluation of medical devices - Part 10:
Tests for irritation and delayed-type hypersensitivity
YY/T 0149-2006 Medical instruments of stainless steel - Test methods of
corrosion resistance
YY 0167-2005 Non-absorbable surgical suture
YY/T 0171-2008 Surgical instruments - Packaging, marking and instructions
Pharmacopoeia of the People's Republic of China (2010 Edition · Part 2)
Example:
Diameter D = 0.7 mm, chord length L = 24 mm, needle length = 31 mm, the
needle hole is a common hole, the form is a round needle, the arc is 3/8 arc,
which is marked as:
Round 3/8 arc • 7 × 24, or, round 3/8 arc • 7 × 24 - 31;
○3/8 arc·7 x 24, or, ○3/8 arc·7 x 24 - 31.
3.4 Materials
The suture needle is made of 30Cr13, 40Cr13, 12Cr18Ni9, 06Cr19Ni10
materials as specified in GB/T 4240, or other materials that meet the
requirements of the standard.
4 Requirements
4.1 Appearance
4.1.1 Needle body
The surface of the suture needle shall be smooth and uniform in color; the
needle tip shall have no hooks; the needle hole and needle groove shall be
smooth and centered; there shall be no burrs or obvious skew.
After the suture needle is connected to the suture thread, the needle tail shall
be smooth and free of burr.
4.1.2 Triangular cutting edge
The cutting edge of the triangular needle shall not be curled, white-edged or
jagged.
4.1.3 Surface roughness
The value of surface roughness Rα shall not be greater than 0.8 μm.
4.1.4 Embedding thread of spring hole
There shall be no defects such as secant (broken strand) or off-line in the
embedding thread of spring hole.
4.2 Physical properties
4.2.1 Hardness
The hardness of the suture needle shall meet the requirements of Table 1.
Carry out testing according to the Appendix XI J Testing Method of Microbial
Limit of the People's Republic of China Pharmacopoeia" (2010 Edition · Part 2),
the results shall comply with the provisions of 4.4.
5.5 Corrosion resistance
The corrosion resistance of the suture needle is tested according to the boiling
water test method of YY/T 0149-2006, the results shall meet the requirements
of 4.5.
5.6 Sterility
Carry out testing according to the Appendix XI H Sterile Test Method of the
People's Republic of China Pharmacopoeia" (2010 Edition · Part 2), the results
shall comply with the provisions of 4.6.
5.7 Residual ethylene oxide
The determination of the residual ethylene oxide is carried out according to the
method specified in GB/T 16886.7-2001, the results shall meet the
requirements of 4.7.
The preparation of the test solution: the suture needle and test water are
prepared in a ratio of 1.0 g:5 mL, sealed and equilibrated at 60 °C ± 1 °C for 40
min.
5.8 Biological evaluation
5.8.1 Carry out according to the method specified in 8.2 (extract liquid test) in
GB/T 16886.5-2003; the results shall meet the requirements of 4.8.1.
Preparation of extract:
a) Extraction conditions (37 ± 1) °C, (24 ± 2) h;
b) Extraction ratio 0.2 g/mL;
c) The extraction medium uses 10% serum.
5.8.2 Carry out according to the method specified in 7.4 (maximum dose test)
in GB/T 16886.10-2005; the results shall meet the requirements of 4.8.2.
5.8.3 Carry out according to the method specified in Appendix B.2 (intradermal
reaction test) in GB/T 16886.10-2005; the results shall meet the requirements
of 4.8.3.
5.9 Packaging and marking
Appendix C
(Normative)
Test method for piercing force and strength of needle tip
C.1 Scope
This method is suitable for testing the needle tip’s piercing force and strength
of suture needles.
C.2 Principle
C.2.1 Evaluate the strength of the suture needle tip by pressing the suture
needle tip vertically against the test material in the specified time under
specified load.
C.2.2 Evaluate the needle tip’s piercing force of the suture needle by the
maximum peak force measured by vertically piercing the test material with the
suture needle at the specified speed.
C.3 Definition
C.3.1
Strength of needles tips
After the needle tip is subject to external force, the force at which hook does
not appear.
C.3.2
Penetration force of needles tips
The force of needle piercing.
C.4 Test equipment
C.4.1 Instrument structure
The instrument structure is as shown in Figure C.1; other instruments with the
same performance and accuracy can also be used (such as adding a
measuring amplifier, data processing and display unit, printer, floppy disk and
other devices).
C.4.2.2 The indication error of the instrument shall not be greater than 0.01 N.
C.4.2.3 The instrument shall be equipped with a level correction and shock-
proof device. The position where the suture needle tips are clamped shall be
adjustable and stable when used.
C.4.2.4 The transmission device of the instrument shall be sensitive and reliable.
When the aluminum foil is pierced in contact with the electrode, the pointer shall
stop automatically.
C.4.2.5 The initial inductance of the instrument shall not be greater than 0.02
N.
C.4.2.6 The aperture of the aluminum foil fixture of the instrument is Φ5 mm;
the aluminum foil after clamping must not be loose; the aluminum foil in the
aperture shall be flat.
C.5 Test materials
C.5.1 Steel block
C.5.1.1 The hardness of the steel block is not less than FRC60.
C.5.1.2 The steel block is a cylinder with a smooth surface and a diamet...
Share