1
/
su
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 0328-2015 English PDF (YYT0328-2015)
YY/T 0328-2015 English PDF (YYT0328-2015)
Prezzo di listino
$150.00 USD
Prezzo di listino
Prezzo scontato
$150.00 USD
Prezzo unitario
/
per
Spese di spedizione calcolate al check-out.
Impossibile caricare la disponibilità di ritiro
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY/T 0328-2015
Historical versions: YY/T 0328-2015
Preview True-PDF (Reload/Scroll if blank)
YY/T 0328-2015: A.V.fistula needle sets for single use
YY/T 0328-2015
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.20
C 31
Replacing YY/T 0328-2002
A.V. fistula needle sets for single use
ISSUED ON. MARCH 02, 2015
IMPLEMENTED ON. JANUARY 01, 2016
Issued by. China Food and Drug Administration
Table of Contents
Foreword . 3
Introduction .. 5
1 Scope .. 6
2 Normative references . 6
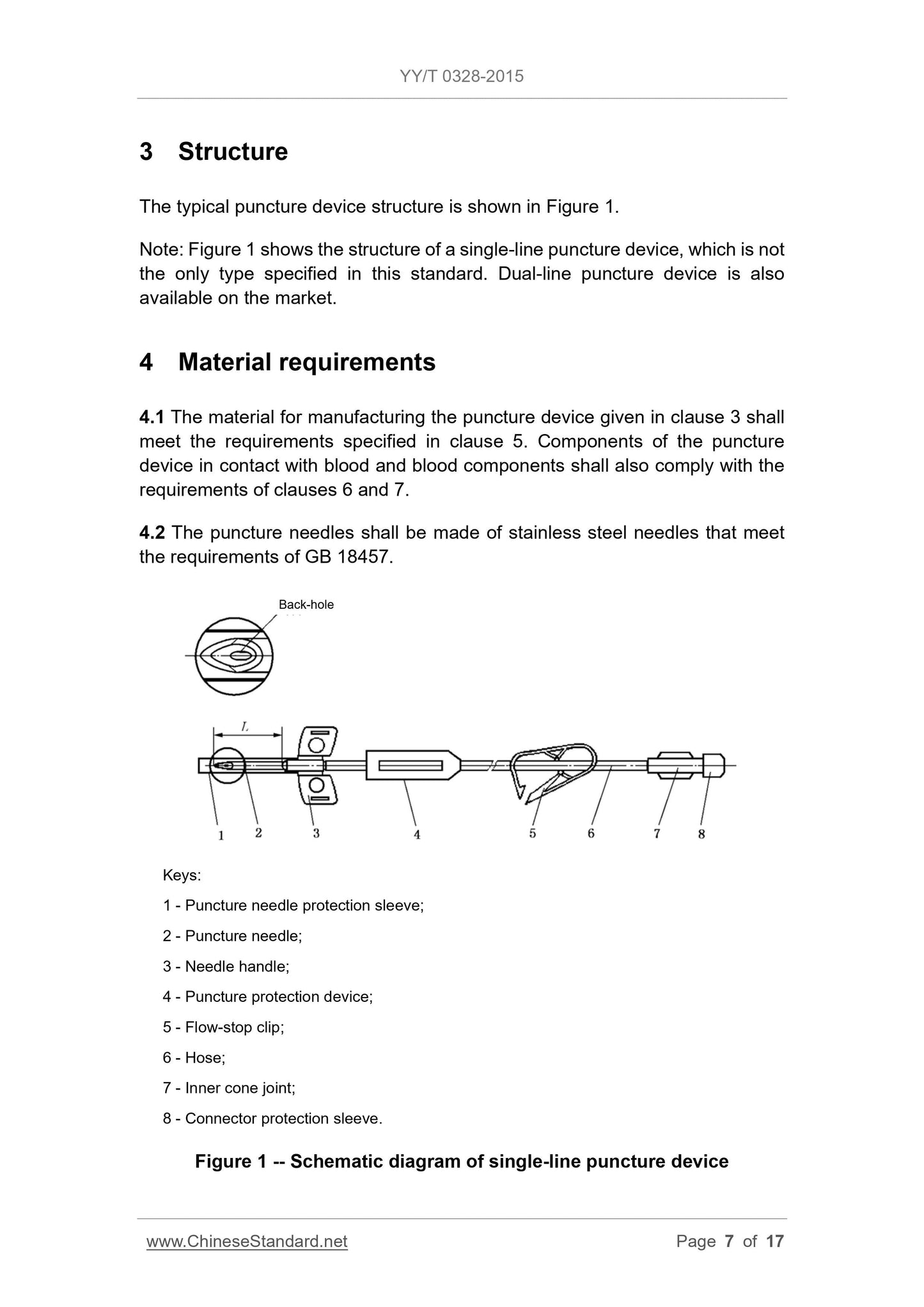
3 Structure . 7
4 Material requirements . 7
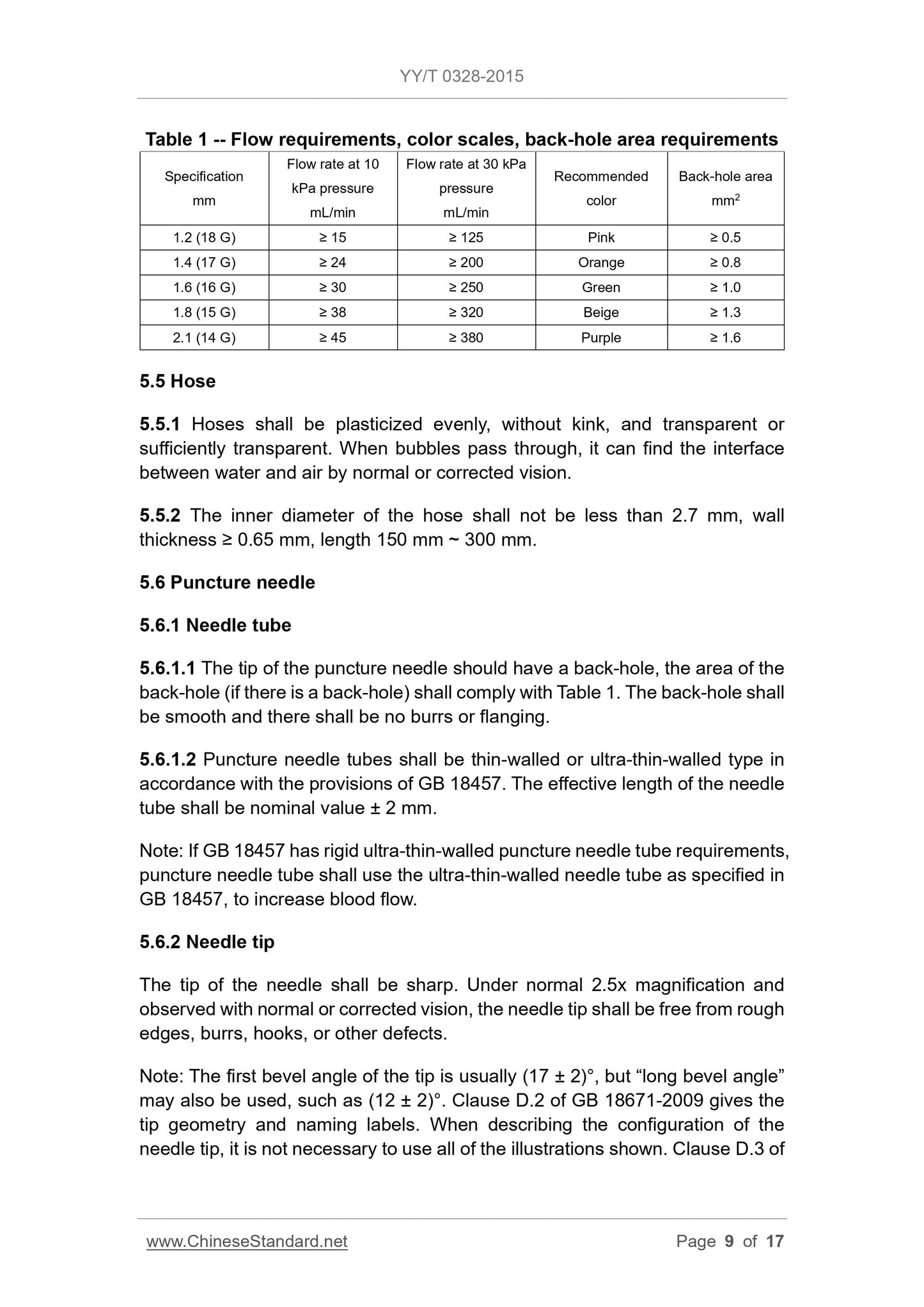
5 Physical requirements . 8
6 Chemical requirements . 11
7 Biological requirements . 12
8 Type test. 13
9 Markings . 13
10 Packaging . 15
Appendix A (Normative) Particle contamination test . 16
References . 17
Foreword
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
Please note that some of the contents of this document may involve patents.
The publication institute of this document does not assume responsibility for
identifying these patents.
This standard replaces YY/T 0328-2002 “Single use blood-taking set for blood
processing equipment”. As compared with YY/T 0328-2002, the main technical
changes of this standard are as follows.
- MODIFY the Chinese and English names of this standard;
- ADD a description of the two-way type;
- CANCEL the description of the product mark;
- MODIFY the requirements for particulate pollution;
- MODIFY the sealing requirements;
- MODIFY the flow rate requirements;
- MODIFY the requirements for puncture needles;
- MODIFY the appearance of the needle handle;
- MODIFY the recommended requirements of needle handle color scales;
- ADD the recommended requirements for stop-flow clip color scales;
- ADD recommended requirements for protection against needle puncture;
- MODIFY the requirements of the pH and test methods;
- MODIFY the requirements for total amount of evaporation residues;
- ADD the requirements that the non-single set package shall be marked of
the quantity and recommended maximum positive and negative pressure.
This standard shall be under the jurisdiction of the National Standardization
Technical Committee for Medical Infusion Devices (SAC/TC 106).
Main drafting organizations of this standard. Shandong Provincial Medical
Device Product Quality Inspection Center.
A.V. fistula needle sets for single use
1 Scope
This standard specifies the requirements for the A.V. fistula needle sets for
single use (hereinafter referred to as puncture devices), to ensure that they are
compatible with the blood flow and blood processing systems that they support.
2 Normative references
The following documents are essential to the application of this document. For
the dated documents, only the versions with the dates indicated are applicable
to this document; for the undated documents, only the latest version (including
all the amendments) are applicable to this standard.
GB/T 1962.2 Conical fittings with a 6% (Luer) taper for syringes, needles and
certain other medical equipment - Part 2. Lock fittings
GB/T 14233.1-2008 Test methods for infusion transfusion injection
equipment for medical use - Part 1. Chemical analysis methods
GB/T 14233.2 Test methods for infusion, transfusion, injection equipment for
medical use - Part 2. Biological test methods
GB/T 16886.1 Biological evaluation of medical devices - Part 1. Evaluation
and testing within a risk management process
GB 18457 Stainless steel needle tubing for the manufacture of medical
devices
GB 18671-2009 Intravenous needles for single use
YY/T 0466.1 Medical devices - Symbols to be used with medical device
labels, labelling and information to be supplied - Part 1. General
requirements
ISO 11607-1.2006 Packaging for terminally sterilized medical devices - Part
1. Requirements for materials, sterile barrier systems and packaging
systems 1)
1 The Chinese standard GB/T 19633.1 equivalent to ISO 11607-1.2006 is currently in the approval stage.
GB 18671-2009 gives the evaluation method of needle tip puncture
performance.
5.6.3 Lubricants
If the needle is coated with a lubricant and observed with the normal or
corrected vision, there shall be no visible accumulation of lubricant on the
outside surface of the needle.
Note. The suitable lubricant is undiluted polydimethylsiloxane in accordance
with national pharmacopoeia. The amount of lubricant per square centimeter of
the surface of the needle shall not exceed 0.25 mg.
5.6.4 Needle handle
5.6.4.1 Appearance
The appearance shall meet the following requirements.
a) The edge of the needle handle shall be complete without burrs;
b) In the appropriate position of the needle handle, it shall have the nominal
outer diameter marking of the needle, the marking shall be clear.
Note. There shall be embossed buckles on the needle edge to facilitate the
fingers to control the angle of the puncture needle.
5.6.4.2 Color scales
The needle handle color should be used to indicate the outer diameter of the
puncture needle. It is recommended to use the color specified in Table 1.
5.6.4.3 Needle handle direction
The needle handle shall be in the same direction as the needle bevel (as shown
in Figure 1).
Note. Movable needles that can rotate axially around the needle handle are not
subject to this requirement, but additional indications of the direction of the
bevel of the needle tip must be provided on the needle handle.
5.7 Flow-stop clip
5.7.1 The flow-stop clip on the puncture device should be a locking pin. The
flow-stop clip shall be able to effectively open and close the hose. When closed,
it shall be able to block the gas that is 50 kPa above atmospheric pressure for
1 min without leakage.
chromium, copper, lead, and tin in the puncture test solution shall not exceed 1
μg/mL. The content of cadmium shall not exceed 0.1 μg/mL.
6.3.2 When tested in accordance with the method of 5.6.1 of GB/T 14233.1-
2008, the color of the puncture device test solution shall not exceed the
standard control solution which has a mass concentration ρ(Pb2+) = 1 μg/mL.
6.4 pH
When tested in accordance with the method of 5.4.2 of GB/T 14233.1-2008,
any standard solution required for graying the indicator shall not exceed 1 mL.
6.5 Evaporation residue
When tested in accordance with them method of 5.5 in GB/T 14233.1-2008, the
total amount of evaporation residues shall not exceed 5 mg.
6.6 UV absorbance
When tested in accordance with the method of 5.7 in GB/T 14233.1-2008, the
absorbance of the puncture test solution in the range of 250 nm ~ 320 nm shall
not exceed 0.1.
6.7 Ethylene oxide residues
When tested in accordance with the methods of clause 9 or clause 10 of GB/T
14233.1-2008, the residual ethylene oxide of each puncture device shall not
exceed 0.5 mg.
7 Biological requirements
7.1 Biocompatibility
The puncture device shall be evaluated biologically in accordance with the
requirements of GB/T 16886.1.
7.2 Asepsis
The puncture device shall undergo a confirmed sterilization process to make
the product sterile.
Note 1. Refer to the reference for suitable sterilization methods.
Note 2. GB/T 14233.2 specifies a sterile test method, but this method should
not be used for exit-factory inspection.
expressed in kPa.
Note. The graphic symbols given in YY/T 0466.1 may be used to satisfy the
above requirements.
9.2 Middle packaging
The middle packaging shall have at least the following clearly identified
informati...
Get QUOTATION in 1-minute: Click YY/T 0328-2015
Historical versions: YY/T 0328-2015
Preview True-PDF (Reload/Scroll if blank)
YY/T 0328-2015: A.V.fistula needle sets for single use
YY/T 0328-2015
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.20
C 31
Replacing YY/T 0328-2002
A.V. fistula needle sets for single use
ISSUED ON. MARCH 02, 2015
IMPLEMENTED ON. JANUARY 01, 2016
Issued by. China Food and Drug Administration
Table of Contents
Foreword . 3
Introduction .. 5
1 Scope .. 6
2 Normative references . 6
3 Structure . 7
4 Material requirements . 7
5 Physical requirements . 8
6 Chemical requirements . 11
7 Biological requirements . 12
8 Type test. 13
9 Markings . 13
10 Packaging . 15
Appendix A (Normative) Particle contamination test . 16
References . 17
Foreword
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
Please note that some of the contents of this document may involve patents.
The publication institute of this document does not assume responsibility for
identifying these patents.
This standard replaces YY/T 0328-2002 “Single use blood-taking set for blood
processing equipment”. As compared with YY/T 0328-2002, the main technical
changes of this standard are as follows.
- MODIFY the Chinese and English names of this standard;
- ADD a description of the two-way type;
- CANCEL the description of the product mark;
- MODIFY the requirements for particulate pollution;
- MODIFY the sealing requirements;
- MODIFY the flow rate requirements;
- MODIFY the requirements for puncture needles;
- MODIFY the appearance of the needle handle;
- MODIFY the recommended requirements of needle handle color scales;
- ADD the recommended requirements for stop-flow clip color scales;
- ADD recommended requirements for protection against needle puncture;
- MODIFY the requirements of the pH and test methods;
- MODIFY the requirements for total amount of evaporation residues;
- ADD the requirements that the non-single set package shall be marked of
the quantity and recommended maximum positive and negative pressure.
This standard shall be under the jurisdiction of the National Standardization
Technical Committee for Medical Infusion Devices (SAC/TC 106).
Main drafting organizations of this standard. Shandong Provincial Medical
Device Product Quality Inspection Center.
A.V. fistula needle sets for single use
1 Scope
This standard specifies the requirements for the A.V. fistula needle sets for
single use (hereinafter referred to as puncture devices), to ensure that they are
compatible with the blood flow and blood processing systems that they support.
2 Normative references
The following documents are essential to the application of this document. For
the dated documents, only the versions with the dates indicated are applicable
to this document; for the undated documents, only the latest version (including
all the amendments) are applicable to this standard.
GB/T 1962.2 Conical fittings with a 6% (Luer) taper for syringes, needles and
certain other medical equipment - Part 2. Lock fittings
GB/T 14233.1-2008 Test methods for infusion transfusion injection
equipment for medical use - Part 1. Chemical analysis methods
GB/T 14233.2 Test methods for infusion, transfusion, injection equipment for
medical use - Part 2. Biological test methods
GB/T 16886.1 Biological evaluation of medical devices - Part 1. Evaluation
and testing within a risk management process
GB 18457 Stainless steel needle tubing for the manufacture of medical
devices
GB 18671-2009 Intravenous needles for single use
YY/T 0466.1 Medical devices - Symbols to be used with medical device
labels, labelling and information to be supplied - Part 1. General
requirements
ISO 11607-1.2006 Packaging for terminally sterilized medical devices - Part
1. Requirements for materials, sterile barrier systems and packaging
systems 1)
1 The Chinese standard GB/T 19633.1 equivalent to ISO 11607-1.2006 is currently in the approval stage.
GB 18671-2009 gives the evaluation method of needle tip puncture
performance.
5.6.3 Lubricants
If the needle is coated with a lubricant and observed with the normal or
corrected vision, there shall be no visible accumulation of lubricant on the
outside surface of the needle.
Note. The suitable lubricant is undiluted polydimethylsiloxane in accordance
with national pharmacopoeia. The amount of lubricant per square centimeter of
the surface of the needle shall not exceed 0.25 mg.
5.6.4 Needle handle
5.6.4.1 Appearance
The appearance shall meet the following requirements.
a) The edge of the needle handle shall be complete without burrs;
b) In the appropriate position of the needle handle, it shall have the nominal
outer diameter marking of the needle, the marking shall be clear.
Note. There shall be embossed buckles on the needle edge to facilitate the
fingers to control the angle of the puncture needle.
5.6.4.2 Color scales
The needle handle color should be used to indicate the outer diameter of the
puncture needle. It is recommended to use the color specified in Table 1.
5.6.4.3 Needle handle direction
The needle handle shall be in the same direction as the needle bevel (as shown
in Figure 1).
Note. Movable needles that can rotate axially around the needle handle are not
subject to this requirement, but additional indications of the direction of the
bevel of the needle tip must be provided on the needle handle.
5.7 Flow-stop clip
5.7.1 The flow-stop clip on the puncture device should be a locking pin. The
flow-stop clip shall be able to effectively open and close the hose. When closed,
it shall be able to block the gas that is 50 kPa above atmospheric pressure for
1 min without leakage.
chromium, copper, lead, and tin in the puncture test solution shall not exceed 1
μg/mL. The content of cadmium shall not exceed 0.1 μg/mL.
6.3.2 When tested in accordance with the method of 5.6.1 of GB/T 14233.1-
2008, the color of the puncture device test solution shall not exceed the
standard control solution which has a mass concentration ρ(Pb2+) = 1 μg/mL.
6.4 pH
When tested in accordance with the method of 5.4.2 of GB/T 14233.1-2008,
any standard solution required for graying the indicator shall not exceed 1 mL.
6.5 Evaporation residue
When tested in accordance with them method of 5.5 in GB/T 14233.1-2008, the
total amount of evaporation residues shall not exceed 5 mg.
6.6 UV absorbance
When tested in accordance with the method of 5.7 in GB/T 14233.1-2008, the
absorbance of the puncture test solution in the range of 250 nm ~ 320 nm shall
not exceed 0.1.
6.7 Ethylene oxide residues
When tested in accordance with the methods of clause 9 or clause 10 of GB/T
14233.1-2008, the residual ethylene oxide of each puncture device shall not
exceed 0.5 mg.
7 Biological requirements
7.1 Biocompatibility
The puncture device shall be evaluated biologically in accordance with the
requirements of GB/T 16886.1.
7.2 Asepsis
The puncture device shall undergo a confirmed sterilization process to make
the product sterile.
Note 1. Refer to the reference for suitable sterilization methods.
Note 2. GB/T 14233.2 specifies a sterile test method, but this method should
not be used for exit-factory inspection.
expressed in kPa.
Note. The graphic symbols given in YY/T 0466.1 may be used to satisfy the
above requirements.
9.2 Middle packaging
The middle packaging shall have at least the following clearly identified
informati...
Share