1
/
su
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1291-2016 English PDF (YYT1291-2016)
YY/T 1291-2016 English PDF (YYT1291-2016)
Prezzo di listino
$150.00 USD
Prezzo di listino
Prezzo scontato
$150.00 USD
Prezzo unitario
/
per
Spese di spedizione calcolate al check-out.
Impossibile caricare la disponibilità di ritiro
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY/T 1291-2016
Historical versions: YY/T 1291-2016
Preview True-PDF (Reload/Scroll if blank)
YY/T 1291-2016: Single use subcutaneous infusion sets for use with insulin pump
YY/T 1291-2016
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.20
C 31
Single use subcutaneous
infusion sets for use with insulin pump
ISSUED ON. MARCH 23, 2016
IMPLEMENTED ON. JANUARY 1, 2017
Issued by. China Food and Drug Administration of China
Table of Contents
Foreword . 3
Introduction .. 4
1 Scope .. 5
2 Normative references . 5
3 Terms and definitions . 6
4 Structure and composition .. 7
5 Materials . 8
6 Physical requirements . 8
7 Chemical properties . 12
8 Biological requirements . 13
9 Type inspection .. 14
10 Marks . 14
11 Packaging . 17
Annex A (Normative) Physical test .. 18
Annex B (Informative) Design guide for subcutaneous infusion set . 21
Bibliography . 23
Single use subcutaneous
infusion sets for use with insulin pump
1 Scope
This Standard specifies the requirements for the subcutaneous infusion set for
use with insulin pump that consists of interface, piping, piercing assembly
(hereinafter referred to as "the subcutaneous infusion set").
This product is a single use sterile product.
This Standard does not include the requirements for insulin-filled devices (e.g.,
drug reservoirs, pre-filled cassette bottles) in insulin pumps.
This Standard does not address the accuracy requirements for flow control
when the subcutaneous infusion set are fitted with an insulin pump.
2 Normative references
The following referenced documents are indispensable for the application of
this document. For dated references, only the edition cited applies. For undated
references, the latest edition of the referenced document (including any
amendments) applies.
GB/T 1962.2, Conical fittings with a 6% (Luer) taper for syringes, needles
and certain other medical equipment - Part 2. Lock fittings (GB/T 1962.2-
2001, ISO 594-2.1998, IDT)
GB 8368-2005, Infusion sets for single use, gravity feed (ISO 8536-4.2004,
MOD)
GB9706.27, Medical electrical equipment - Part 2. Particular requirements
for the safety of infusion pumps and controllers (GB 9706.27-2005, IEC
60601-2-24.1998, IDT)
GB/T 14233.1-2008, Test methods for infusion transfusion injection
equipment for medical use - Part 1. Chemical analysis methods
GB/T 14233.2-2005, Test methods for infusion, transfusion, injection
equipment for medical use - Part 2. Biological test methods
GB/T 16886.1, Biological evaluation of medical devices - Part 1. Evaluation
and testing (GB/T 16886.1-2011, ISO 10993-1.2009, IDT)
GB 18457, Stainless steel needle tubing for the manufacture of medical
devices (GB 18457-2001, ISO 9626.1991, IDT)
YY/T 0148-2006, Medical adhesive bandages - General requirements
YY 0285.1-2004, Sterile, single-use intravascular catheters - Part 1. General
requirements (ISO 10555-1.1995, IDT)
YY 0285.5-2004, Sterile, single-use intravascular catheters - Part 5. Over-
needle peripheral catheters (ISO 10555-5.1996, IDT)
YY/T 0466.1, Medical devices - Symbols to be used with medical device
labels, labelling and information to be supplied - Part 1. General
requirements (YY/T 0466.1-2009, ISO 15223-1.2007, IDT)
YY/T 0615.1, Requirements for medical devices to be designated STERILE
- Part 1. Requirements for terminally sterilized medical devices
ISO 11607-1.2006, Packaging for terminally sterilized medical devices - Part
1. Requirements for materials, sterile barrier systems and packaging
systems1
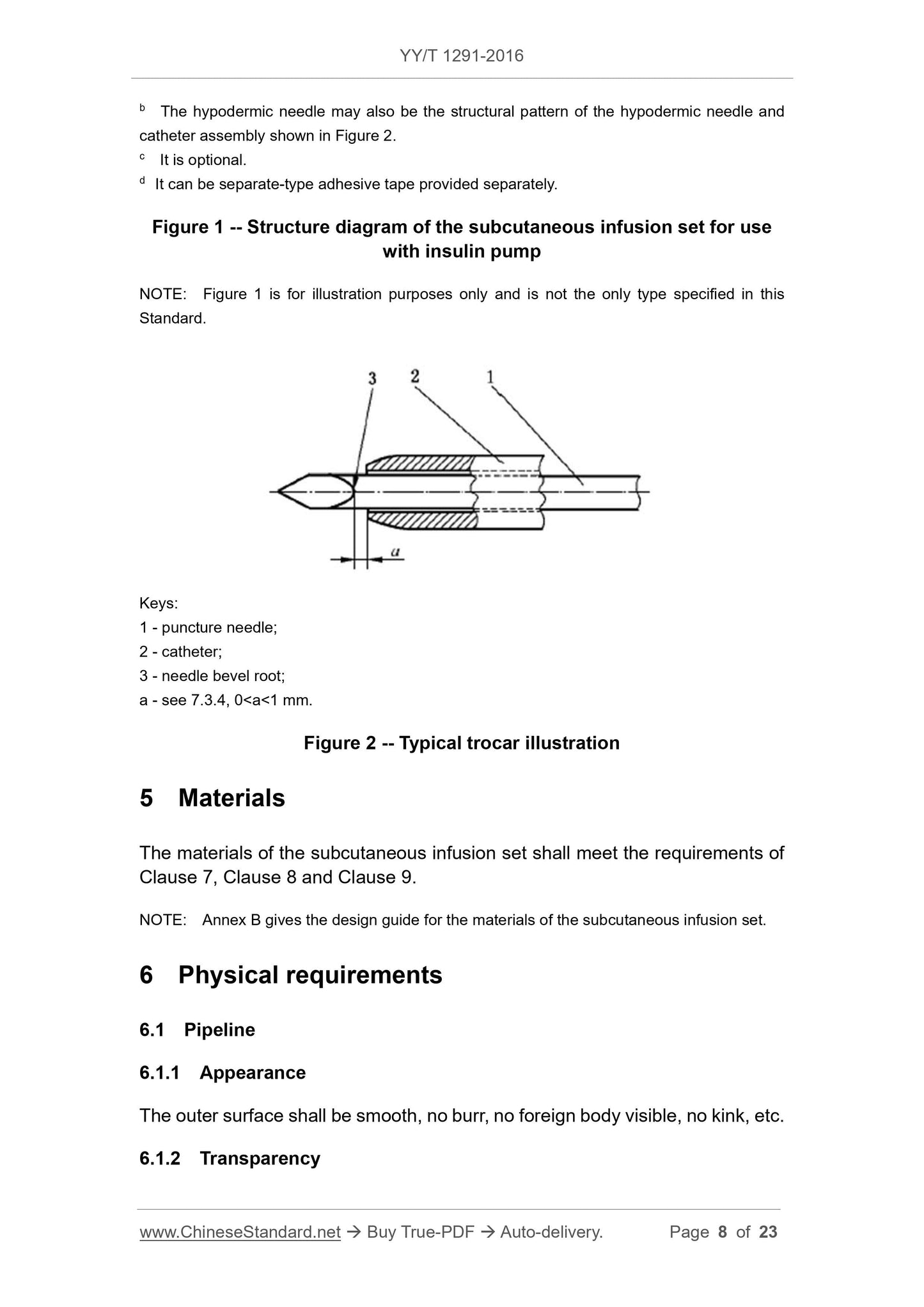
3 Terms and definitions
For the purposes of this document, the following terms and definitions apply.
3.1 introducer needle unit
a unit on the subcutaneous infusion set for subcutaneous puncture, usually
consisting of hypodermic needle (or hypodermic needle and catheter assembly),
needle handle (or catheter base), adhesive tape for fixing use.
3.2 hypodermic needle
a rigid tube with cutting edge on one end for subcutaneous administration; it
generally has oblique insertion type and in-line type
3.3 hypodermic needle and catheter assembly
an assembly that consists of a metal piercing needle and a catheter over it; the
hypodermic needle and catheter assembly punctures the skin, the metal
puncture needle is pulled out, catheter is indwelled subcutaneously for
subcutaneous administration
1 The Chinese standard GB/T 19633.1 that is equivalent to ISO11607-1.2006 is currently in the
approval stage.
It shall be transparent or sufficiently transparent. When tested according to A.1,
it shall be able to detect the gas-liquid interface.
6.1.3 Length
The pipeline length shall not be less than 90% of the nominal value.
6.2 Hypodermic needle (if any)
6.2.1 Needle tube
The needle section and wall thickness shall be uniform. The liquid passage of
the needle shall be unimpeded.
6.2.2 Needle tube length
The effective length of the needle tube shall meet the requirements of the
nominal value, the tolerance is ±1mm.
NOTE. The effective length of the needle is the length of the first bend from the needle tip to
the needle tube. For in-line hypodermic needle, the first bend is the junction of the needle tube
and the introducer needle unit adhesive tape, i.e. the bending angle is close to 90°. For oblique
insertion type hypodermic needle, the first bend is exposed at the outside bend.
6.2.3 Bending direction and angle of needle tube
When the direction is parallel to the fixed surface of the needle handle and the
direction of the pipeline (in the direction shown in Figure 1), it is visually
observing the bending angle of the needle tube, the included angle between
the needle tube and the tube side on the needle stem shall be equal to or
greater than 90°.
6.2.4 Needle tip
With normal vision or corrected vision, the needle tip shall be sharp, free from
kinks, nicks, and hooks when examined under magnification 2.5 times.
6.2.5 Connection firmness of needle handle
At the connection between hypodermic needle and needle handle, apply 10N
of axial static tensile force for 15s. It shall not be disconnected or loosened.
6.3 Hypodermic needle and catheter assembly (if any)
6.3.1 Catheter length
The effective length of the catheter (the length from the tip of the catheter to the
end of the catheter) shall be expressed in millimeters. The effective length of
the catheter shall meet the requirements of the nominal value, the tolerance is
When tested according to 5.2.2 in GB/T 14233.1-2008, the difference between
the volume of potassium permanganate solution c(KMnO4)=0.002mol/L
consumed by test solution and blank solution shall not exceed 2.0 mL.
7.3 Metal ion
When tested in 5.9.1 atomic absorption spectrophotometry (AAS) or the
equivalent method in GB/T 14233.1-2008, the total content of antimony,
chromium, copper, lead, and tin in the test liquid shall not exceed 1 μg/mL, and
the content of cadmium not exceed 0.1 μg/mL.
When tested according to the colorimetric method in 5.6.1 of GB/T 14233.1-
2008, the color of the test liquid shall not exceed the standard control solution
with a mass concentration ρ(Pb2+)=1 μg/mL.
7.4 Acidity titration
When tested according to the method of 5.4.2 of GB/T 14233.1-2008, any
standard solution required to gray the color of the indicator shall not exceed 1
mL.
7.5 Evaporation residue
When tested according to the method of 5.5 in GB/T 14233.1-2008, the total
amount of evaporation residue shall not exceed 2 mg.
7.6 UV absorbance
When tested according to the method of 5.7 in GB/T 14233.1-...
Get QUOTATION in 1-minute: Click YY/T 1291-2016
Historical versions: YY/T 1291-2016
Preview True-PDF (Reload/Scroll if blank)
YY/T 1291-2016: Single use subcutaneous infusion sets for use with insulin pump
YY/T 1291-2016
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.20
C 31
Single use subcutaneous
infusion sets for use with insulin pump
ISSUED ON. MARCH 23, 2016
IMPLEMENTED ON. JANUARY 1, 2017
Issued by. China Food and Drug Administration of China
Table of Contents
Foreword . 3
Introduction .. 4
1 Scope .. 5
2 Normative references . 5
3 Terms and definitions . 6
4 Structure and composition .. 7
5 Materials . 8
6 Physical requirements . 8
7 Chemical properties . 12
8 Biological requirements . 13
9 Type inspection .. 14
10 Marks . 14
11 Packaging . 17
Annex A (Normative) Physical test .. 18
Annex B (Informative) Design guide for subcutaneous infusion set . 21
Bibliography . 23
Single use subcutaneous
infusion sets for use with insulin pump
1 Scope
This Standard specifies the requirements for the subcutaneous infusion set for
use with insulin pump that consists of interface, piping, piercing assembly
(hereinafter referred to as "the subcutaneous infusion set").
This product is a single use sterile product.
This Standard does not include the requirements for insulin-filled devices (e.g.,
drug reservoirs, pre-filled cassette bottles) in insulin pumps.
This Standard does not address the accuracy requirements for flow control
when the subcutaneous infusion set are fitted with an insulin pump.
2 Normative references
The following referenced documents are indispensable for the application of
this document. For dated references, only the edition cited applies. For undated
references, the latest edition of the referenced document (including any
amendments) applies.
GB/T 1962.2, Conical fittings with a 6% (Luer) taper for syringes, needles
and certain other medical equipment - Part 2. Lock fittings (GB/T 1962.2-
2001, ISO 594-2.1998, IDT)
GB 8368-2005, Infusion sets for single use, gravity feed (ISO 8536-4.2004,
MOD)
GB9706.27, Medical electrical equipment - Part 2. Particular requirements
for the safety of infusion pumps and controllers (GB 9706.27-2005, IEC
60601-2-24.1998, IDT)
GB/T 14233.1-2008, Test methods for infusion transfusion injection
equipment for medical use - Part 1. Chemical analysis methods
GB/T 14233.2-2005, Test methods for infusion, transfusion, injection
equipment for medical use - Part 2. Biological test methods
GB/T 16886.1, Biological evaluation of medical devices - Part 1. Evaluation
and testing (GB/T 16886.1-2011, ISO 10993-1.2009, IDT)
GB 18457, Stainless steel needle tubing for the manufacture of medical
devices (GB 18457-2001, ISO 9626.1991, IDT)
YY/T 0148-2006, Medical adhesive bandages - General requirements
YY 0285.1-2004, Sterile, single-use intravascular catheters - Part 1. General
requirements (ISO 10555-1.1995, IDT)
YY 0285.5-2004, Sterile, single-use intravascular catheters - Part 5. Over-
needle peripheral catheters (ISO 10555-5.1996, IDT)
YY/T 0466.1, Medical devices - Symbols to be used with medical device
labels, labelling and information to be supplied - Part 1. General
requirements (YY/T 0466.1-2009, ISO 15223-1.2007, IDT)
YY/T 0615.1, Requirements for medical devices to be designated STERILE
- Part 1. Requirements for terminally sterilized medical devices
ISO 11607-1.2006, Packaging for terminally sterilized medical devices - Part
1. Requirements for materials, sterile barrier systems and packaging
systems1
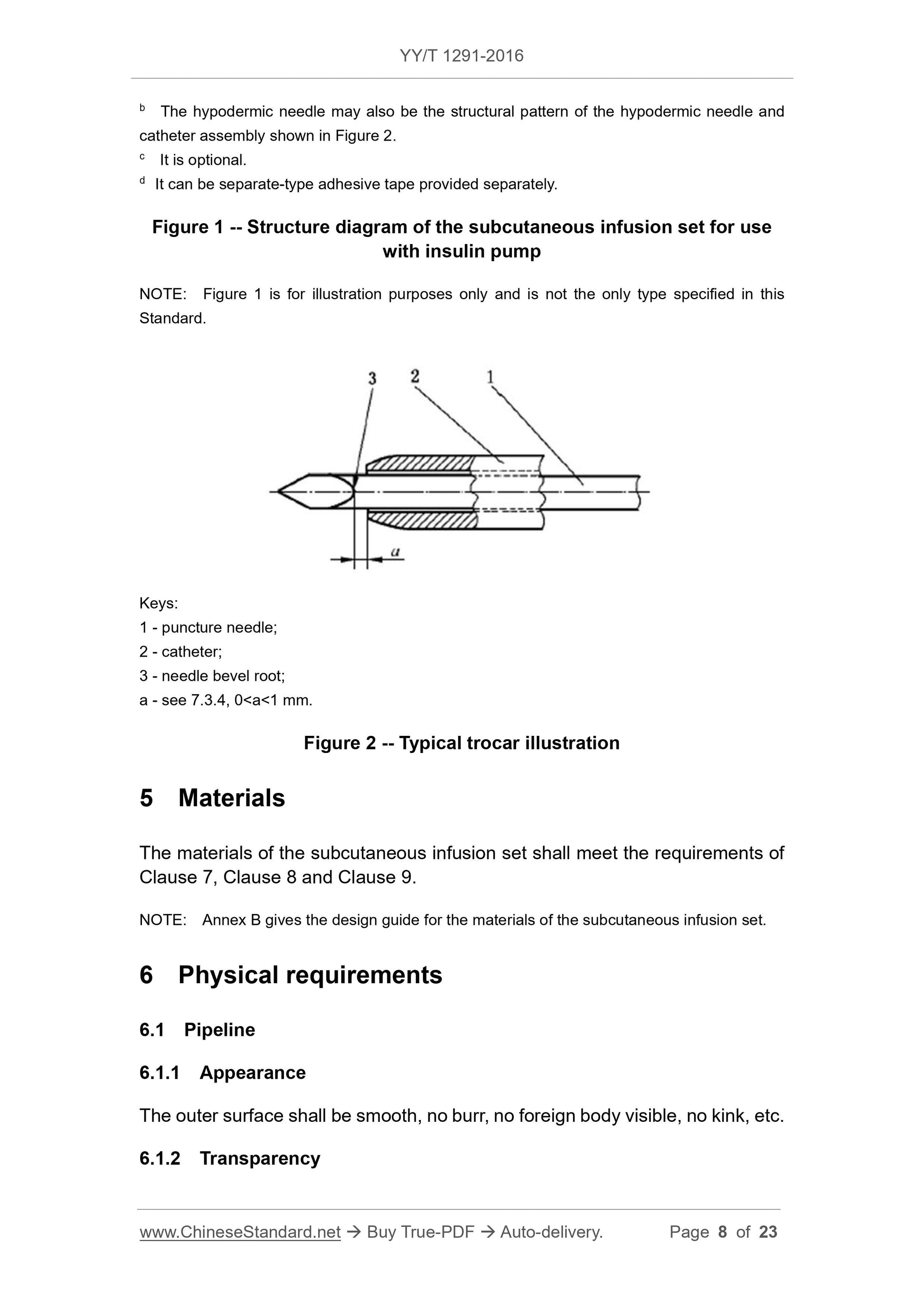
3 Terms and definitions
For the purposes of this document, the following terms and definitions apply.
3.1 introducer needle unit
a unit on the subcutaneous infusion set for subcutaneous puncture, usually
consisting of hypodermic needle (or hypodermic needle and catheter assembly),
needle handle (or catheter base), adhesive tape for fixing use.
3.2 hypodermic needle
a rigid tube with cutting edge on one end for subcutaneous administration; it
generally has oblique insertion type and in-line type
3.3 hypodermic needle and catheter assembly
an assembly that consists of a metal piercing needle and a catheter over it; the
hypodermic needle and catheter assembly punctures the skin, the metal
puncture needle is pulled out, catheter is indwelled subcutaneously for
subcutaneous administration
1 The Chinese standard GB/T 19633.1 that is equivalent to ISO11607-1.2006 is currently in the
approval stage.
It shall be transparent or sufficiently transparent. When tested according to A.1,
it shall be able to detect the gas-liquid interface.
6.1.3 Length
The pipeline length shall not be less than 90% of the nominal value.
6.2 Hypodermic needle (if any)
6.2.1 Needle tube
The needle section and wall thickness shall be uniform. The liquid passage of
the needle shall be unimpeded.
6.2.2 Needle tube length
The effective length of the needle tube shall meet the requirements of the
nominal value, the tolerance is ±1mm.
NOTE. The effective length of the needle is the length of the first bend from the needle tip to
the needle tube. For in-line hypodermic needle, the first bend is the junction of the needle tube
and the introducer needle unit adhesive tape, i.e. the bending angle is close to 90°. For oblique
insertion type hypodermic needle, the first bend is exposed at the outside bend.
6.2.3 Bending direction and angle of needle tube
When the direction is parallel to the fixed surface of the needle handle and the
direction of the pipeline (in the direction shown in Figure 1), it is visually
observing the bending angle of the needle tube, the included angle between
the needle tube and the tube side on the needle stem shall be equal to or
greater than 90°.
6.2.4 Needle tip
With normal vision or corrected vision, the needle tip shall be sharp, free from
kinks, nicks, and hooks when examined under magnification 2.5 times.
6.2.5 Connection firmness of needle handle
At the connection between hypodermic needle and needle handle, apply 10N
of axial static tensile force for 15s. It shall not be disconnected or loosened.
6.3 Hypodermic needle and catheter assembly (if any)
6.3.1 Catheter length
The effective length of the catheter (the length from the tip of the catheter to the
end of the catheter) shall be expressed in millimeters. The effective length of
the catheter shall meet the requirements of the nominal value, the tolerance is
When tested according to 5.2.2 in GB/T 14233.1-2008, the difference between
the volume of potassium permanganate solution c(KMnO4)=0.002mol/L
consumed by test solution and blank solution shall not exceed 2.0 mL.
7.3 Metal ion
When tested in 5.9.1 atomic absorption spectrophotometry (AAS) or the
equivalent method in GB/T 14233.1-2008, the total content of antimony,
chromium, copper, lead, and tin in the test liquid shall not exceed 1 μg/mL, and
the content of cadmium not exceed 0.1 μg/mL.
When tested according to the colorimetric method in 5.6.1 of GB/T 14233.1-
2008, the color of the test liquid shall not exceed the standard control solution
with a mass concentration ρ(Pb2+)=1 μg/mL.
7.4 Acidity titration
When tested according to the method of 5.4.2 of GB/T 14233.1-2008, any
standard solution required to gray the color of the indicator shall not exceed 1
mL.
7.5 Evaporation residue
When tested according to the method of 5.5 in GB/T 14233.1-2008, the total
amount of evaporation residue shall not exceed 2 mg.
7.6 UV absorbance
When tested according to the method of 5.7 in GB/T 14233.1-...
Share