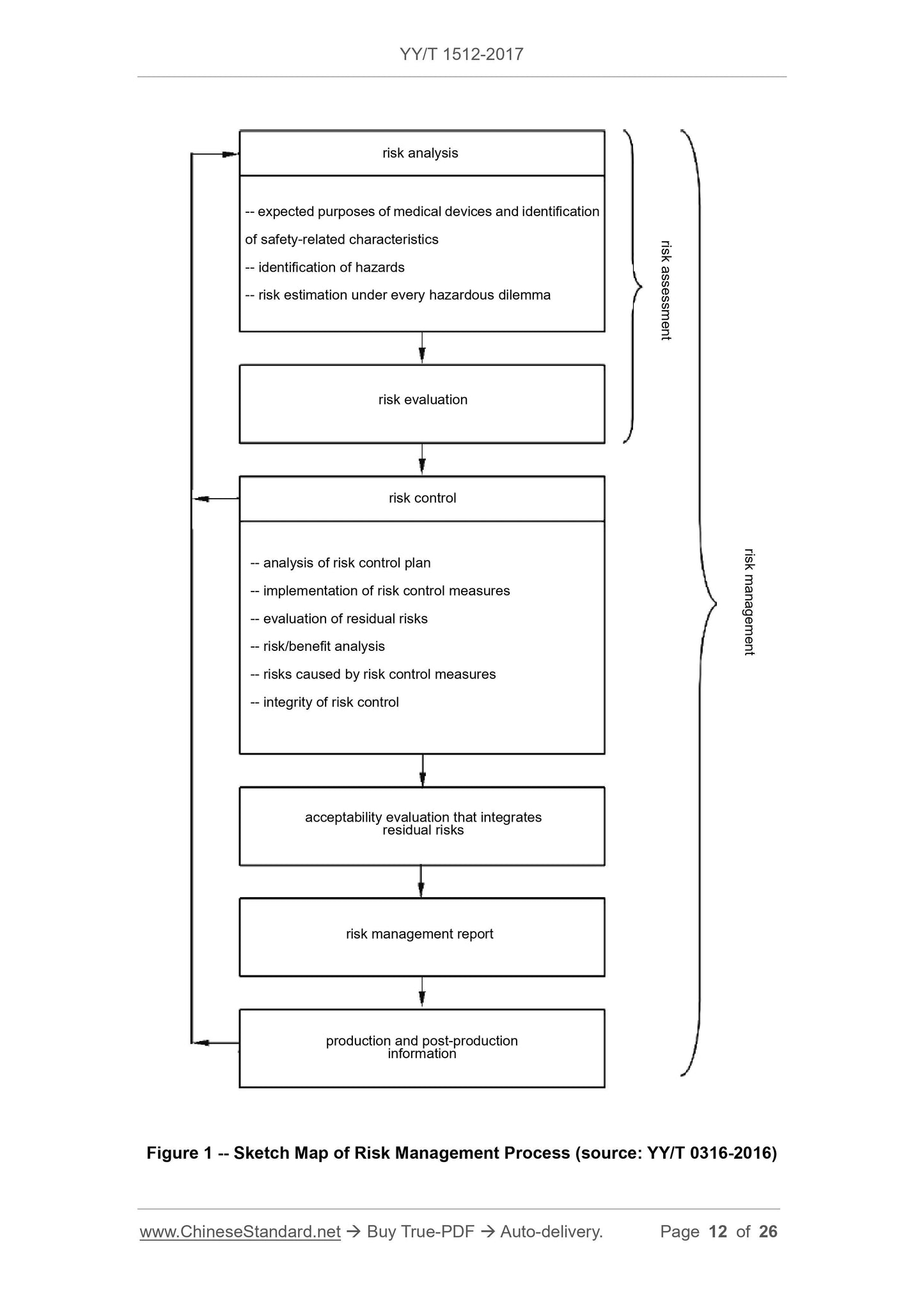
1
/
su
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1512-2017 English PDF (YYT1512-2017)
YY/T 1512-2017 English PDF (YYT1512-2017)
Prezzo di listino
$145.00 USD
Prezzo di listino
Prezzo scontato
$145.00 USD
Prezzo unitario
/
per
Spese di spedizione calcolate al check-out.
Impossibile caricare la disponibilità di ritiro
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY/T 1512-2017
Historical versions: YY/T 1512-2017
Preview True-PDF (Reload/Scroll if blank)
YY/T 1512-2017: Biological evaluation of medical devices—Guidance on the conduct of biological evaluation within a risk management process
YY/T 1512-2017
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.01
C 30
Biological Evaluation of Medical Devices -
Guidance on the Conduct of Biological
Evaluation within a Risk Management Process
(ISO/TR 15499.2016, MOD)
ISSUED ON. JULY 17, 2017
IMPLEMENTED ON. JULY 1, 2018
Issued by. China Food and Drug Administration
Table of Contents
Foreword ... 3
Introduction ... 5
1 Scope ... 7
2 Normative References ... 7
3 Terms and Definitions ... 8
4 Biological Evaluation is a Risk Management Activity ... 9
5 Risk Management Guidance ... 13
6 Guidance of Biological Evaluation in Specific Aspects ... 17
References ... 25
Foreword
This Standard is drafted in accordance with stipulations in GB/T 1.1-2009.
This Standard is modified in accordance with the Law of Redrafting on the basis of
ISO/TR 15499.2016 Biological Evaluation of Medical Devices - Guidance on the
Conduct of Biological Evaluation within a Risk Management Process.
In comparison with ISO/TR 15499.2016, there are several technical differences and
causes for these differences as follows.
-- In terms of normative references, there is an adjustment of technical differences
in this Standard to comply with national-level technical conditions. The condition
of such adjustment is intensively reflected in Chapter 2 “Normative References”.
Please see the specific adjustment below.
GB/T 16886.1-2011, which adopts equivalent international standards, is
adopted to replace ISO 10993-1.2009;
GB/T 16886.7, which adopts equivalent international standards, is adopted to
replace ISO 10993-7;
GB/T 16886.9, which adopts equivalent international standards, is adopted to
replace ISO 10993-9;
GB/T 16886.12, which adopts equivalent international standards, is adopted to
replace ISO 10993-12;
GB/T 16886.13, which adopts equivalent international standards, is adopted to
replace ISO 10993-13;
GB/T 16886.14, which adopts equivalent international standards, is adopted to
replace ISO 10993-14;
GB/T 16886.15, which adopts equivalent international standards, is adopted to
replace ISO 10993-15;
GB/T 16886.16, which adopts equivalent international standards, is adopted to
replace ISO 10993-16;
GB/T 16886.17, which adopts equivalent international standards, is adopted to
replace ISO 10993-17;
GB/T 16886.18, which adopts equivalent international standards, is adopted to
replace ISO 10993-18;
GB/T 16886.19, which adopts equivalent international standards, is adopted to
replace ISO 10993-19;
YY/T 0287-2017, which adopts equivalent international standards, is adopted
to replace ISO 13485.2016;
YY/T 0316-2016, which adopts equivalent international standards, is adopted
to replace ISO 14971.2007;
CNAS-CL01, which adopts equivalent international standards, is adopted to
replace ISO/IEC 17025.
Please be noted that certain content in this Standard might involve patents. The
institution that issues this Standard shall not undertake any responsibility of identifying
these patents.
This Standard is proposed by State Food and Drug Administration of the People’s
Republic of China.
This Standard is summarized by National Technical Committee 248 on Medical Device
Biological Evaluation Standardization (SAC/TC 248).
This Standard is drafted by. Shandong Quality Inspection Center for Medical Devices.
The main drafters of this Standard include. Liu Chenghu, Houli, Wuping.
Introduction
0.1 General Principles
This Standard provides guidance on the conduct of biological evaluation of medical
devices in accordance with the requirements in GB/T 16886.1-2011. Although GB/T
16886.1-2011 provides a general framework to the biological evaluation of medical
devices, more specific guidance is necessary during the practical application of this
Standard. Therefore, this Standard is formulated to provide guidance to users of GB/T
16886.1-2011. This Standard facilitates better understanding of the requirements in
GB/T 16886.1-2011 and elaborates various modes and methods that comply with the
requirements in GB/T 16886.1-2011.
Biological evaluation is a group of relatively broad design and verification activities
within the category of risk management process. Therefore, this Standard includes the
guidance of applying GB/T 16886.1-2011 during the risk management process in
accordance with the requirements in YY/T 0316-2016. As a part of the general
evaluation and development of medical devices, concepts and methods described in
this Standard can be taken into consideration during the establishment and
maintenance of a risk management process of biological evaluation.
With the scientific development, we have continuously intensified our grasp of the
fundamental mechanism of tissue reaction. Biological evaluation can be established
on the basis of the review of relevant scientific data, chemical analysis and necessary
in-vivo and in-vitro test. GB/T 16886.1-2011 stipulates a framework of planning
biological evaluation. Through the preferred adoption of chemical components, and in-
vitro models that can obtain equivalent relevant information with in-vivo models, the
quantity of experimental animals and the degree of exposure are controlled to the
minimum. The selection of an appropriate method for a specific medical device will
depend on the characteristics of the device, the availability of of relevant scientific data
and risk assessment.
During the assessment of the availability of the guidance in this Standard, the
requirements and guidance of applicable laws and regulations shall be taken into
consideration.
Organization groups can voluntarily include the whole or any part of the guidance in
this Standard in their risk management process.
The guidance included in this Standard can be assessed as background information
by assessors in the risk management process in institutions and regulatory authorities.
0.2 The Relations Among other Standards, Guidance Standards and
Regulatory Requirements
The relations among GB/T 16886.1-2011, this Standard, and medical device biological
Biological Evaluation of Medical Devices -
Guidance on the Conduct of Biological
Evaluation within a Risk Management Process
1 Scope
This Standard is applicable to the biological evaluation of medical devices in
accordance with the requirements in GB/T 16886.1-2011. This Standard does not add
or modify the requirements in GB/T 16886.1-2011. This Standard does not include
requirements of regulatory inspection or certification and assessment activities.
This Standard is applicable to all the biological evaluation of various types of medical
devices, including active, passive, implantable and non-implantable medical devices.
2 Normative References
The following documents are indispensable to the application of this Standard. In terms
of references with a specified date, only versions with a specified date are applicable
to this Standard. The latest version (including all the modifications) of references
without a specified date is also applicable to this Standard.
GB/T 16886.1-2011 Biological Evaluation of Medical Devices - Part 1. Evaluation
and Testing within a Risk Management Process (ISO 10993-1.2009, IDT)
GB/T 16886.7 Biological Evaluation of Medical Devices - Part 7. Ethylene Oxide
Sterilization Residuals (GB/T 16886.7-2015, ISO 10993-7.2008, IDT)
GB/T 16886.9 Biological Evaluation of Medical Devices -...
Get QUOTATION in 1-minute: Click YY/T 1512-2017
Historical versions: YY/T 1512-2017
Preview True-PDF (Reload/Scroll if blank)
YY/T 1512-2017: Biological evaluation of medical devices—Guidance on the conduct of biological evaluation within a risk management process
YY/T 1512-2017
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.01
C 30
Biological Evaluation of Medical Devices -
Guidance on the Conduct of Biological
Evaluation within a Risk Management Process
(ISO/TR 15499.2016, MOD)
ISSUED ON. JULY 17, 2017
IMPLEMENTED ON. JULY 1, 2018
Issued by. China Food and Drug Administration
Table of Contents
Foreword ... 3
Introduction ... 5
1 Scope ... 7
2 Normative References ... 7
3 Terms and Definitions ... 8
4 Biological Evaluation is a Risk Management Activity ... 9
5 Risk Management Guidance ... 13
6 Guidance of Biological Evaluation in Specific Aspects ... 17
References ... 25
Foreword
This Standard is drafted in accordance with stipulations in GB/T 1.1-2009.
This Standard is modified in accordance with the Law of Redrafting on the basis of
ISO/TR 15499.2016 Biological Evaluation of Medical Devices - Guidance on the
Conduct of Biological Evaluation within a Risk Management Process.
In comparison with ISO/TR 15499.2016, there are several technical differences and
causes for these differences as follows.
-- In terms of normative references, there is an adjustment of technical differences
in this Standard to comply with national-level technical conditions. The condition
of such adjustment is intensively reflected in Chapter 2 “Normative References”.
Please see the specific adjustment below.
GB/T 16886.1-2011, which adopts equivalent international standards, is
adopted to replace ISO 10993-1.2009;
GB/T 16886.7, which adopts equivalent international standards, is adopted to
replace ISO 10993-7;
GB/T 16886.9, which adopts equivalent international standards, is adopted to
replace ISO 10993-9;
GB/T 16886.12, which adopts equivalent international standards, is adopted to
replace ISO 10993-12;
GB/T 16886.13, which adopts equivalent international standards, is adopted to
replace ISO 10993-13;
GB/T 16886.14, which adopts equivalent international standards, is adopted to
replace ISO 10993-14;
GB/T 16886.15, which adopts equivalent international standards, is adopted to
replace ISO 10993-15;
GB/T 16886.16, which adopts equivalent international standards, is adopted to
replace ISO 10993-16;
GB/T 16886.17, which adopts equivalent international standards, is adopted to
replace ISO 10993-17;
GB/T 16886.18, which adopts equivalent international standards, is adopted to
replace ISO 10993-18;
GB/T 16886.19, which adopts equivalent international standards, is adopted to
replace ISO 10993-19;
YY/T 0287-2017, which adopts equivalent international standards, is adopted
to replace ISO 13485.2016;
YY/T 0316-2016, which adopts equivalent international standards, is adopted
to replace ISO 14971.2007;
CNAS-CL01, which adopts equivalent international standards, is adopted to
replace ISO/IEC 17025.
Please be noted that certain content in this Standard might involve patents. The
institution that issues this Standard shall not undertake any responsibility of identifying
these patents.
This Standard is proposed by State Food and Drug Administration of the People’s
Republic of China.
This Standard is summarized by National Technical Committee 248 on Medical Device
Biological Evaluation Standardization (SAC/TC 248).
This Standard is drafted by. Shandong Quality Inspection Center for Medical Devices.
The main drafters of this Standard include. Liu Chenghu, Houli, Wuping.
Introduction
0.1 General Principles
This Standard provides guidance on the conduct of biological evaluation of medical
devices in accordance with the requirements in GB/T 16886.1-2011. Although GB/T
16886.1-2011 provides a general framework to the biological evaluation of medical
devices, more specific guidance is necessary during the practical application of this
Standard. Therefore, this Standard is formulated to provide guidance to users of GB/T
16886.1-2011. This Standard facilitates better understanding of the requirements in
GB/T 16886.1-2011 and elaborates various modes and methods that comply with the
requirements in GB/T 16886.1-2011.
Biological evaluation is a group of relatively broad design and verification activities
within the category of risk management process. Therefore, this Standard includes the
guidance of applying GB/T 16886.1-2011 during the risk management process in
accordance with the requirements in YY/T 0316-2016. As a part of the general
evaluation and development of medical devices, concepts and methods described in
this Standard can be taken into consideration during the establishment and
maintenance of a risk management process of biological evaluation.
With the scientific development, we have continuously intensified our grasp of the
fundamental mechanism of tissue reaction. Biological evaluation can be established
on the basis of the review of relevant scientific data, chemical analysis and necessary
in-vivo and in-vitro test. GB/T 16886.1-2011 stipulates a framework of planning
biological evaluation. Through the preferred adoption of chemical components, and in-
vitro models that can obtain equivalent relevant information with in-vivo models, the
quantity of experimental animals and the degree of exposure are controlled to the
minimum. The selection of an appropriate method for a specific medical device will
depend on the characteristics of the device, the availability of of relevant scientific data
and risk assessment.
During the assessment of the availability of the guidance in this Standard, the
requirements and guidance of applicable laws and regulations shall be taken into
consideration.
Organization groups can voluntarily include the whole or any part of the guidance in
this Standard in their risk management process.
The guidance included in this Standard can be assessed as background information
by assessors in the risk management process in institutions and regulatory authorities.
0.2 The Relations Among other Standards, Guidance Standards and
Regulatory Requirements
The relations among GB/T 16886.1-2011, this Standard, and medical device biological
Biological Evaluation of Medical Devices -
Guidance on the Conduct of Biological
Evaluation within a Risk Management Process
1 Scope
This Standard is applicable to the biological evaluation of medical devices in
accordance with the requirements in GB/T 16886.1-2011. This Standard does not add
or modify the requirements in GB/T 16886.1-2011. This Standard does not include
requirements of regulatory inspection or certification and assessment activities.
This Standard is applicable to all the biological evaluation of various types of medical
devices, including active, passive, implantable and non-implantable medical devices.
2 Normative References
The following documents are indispensable to the application of this Standard. In terms
of references with a specified date, only versions with a specified date are applicable
to this Standard. The latest version (including all the modifications) of references
without a specified date is also applicable to this Standard.
GB/T 16886.1-2011 Biological Evaluation of Medical Devices - Part 1. Evaluation
and Testing within a Risk Management Process (ISO 10993-1.2009, IDT)
GB/T 16886.7 Biological Evaluation of Medical Devices - Part 7. Ethylene Oxide
Sterilization Residuals (GB/T 16886.7-2015, ISO 10993-7.2008, IDT)
GB/T 16886.9 Biological Evaluation of Medical Devices -...
Share