1
/
su
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1671-2020 English PDF (YYT1671-2020)
YY/T 1671-2020 English PDF (YYT1671-2020)
Prezzo di listino
$175.00 USD
Prezzo di listino
Prezzo scontato
$175.00 USD
Prezzo unitario
/
per
Spese di spedizione calcolate al check-out.
Impossibile caricare la disponibilità di ritiro
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY/T 1671-2020
Historical versions: YY/T 1671-2020
Preview True-PDF (Reload/Scroll if blank)
YY/T 1671-2020: Ultrasound biopsy guide
YY/T 1671-2020
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.50
C 41
Ultrasound biopsy guide
超声探头穿刺架
ISSUED ON: FEBRUARY 25, 2020
IMPLEMENTED ON: MARCH 01, 2022
Issued by: National Medical Products Administration
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative references ... 4
3 Terms and definitions ... 4
4 Structure type and nomenclature ... 5
5 Requirements... 5
6 Test methods ... 7
Appendix A (Informative) Typical types of biopsy guide structure ... 13
Ultrasound biopsy guide
1 Scope
This Standard specifies the terms and definitions, structure type and
nomenclature, requirements, and test methods for ultrasound biopsy guide.
This Standard applies to the ultrasound biopsy guide.
This Standard does not apply to inductive navigation devices, such as magnetic
navigation devices.
2 Normative references
The following documents are indispensable for the application of this document.
For the dated references, only the editions with the dates indicated are
applicable to this document. For the undated references, the latest edition
(including all the amendments) are applicable to this document.
GB/T 14233.1-2008 Test methods for infusion, transfusion, injection
equipment for medical use - Part 1: Chemical analysis methods
GB/T 16886.1 Biological evaluation of medical devices - Part 1: Evaluation
and testing within a risk management process
Pharmacopoeia of the People's Republic of China (2015)
3 Terms and definitions
The following terms and definitions apply to this document.
3.1
Ultrasound biopsy guide
A device used for guiding and fixing puncture instruments such as puncture
needles, drainage tubes, treatment or dosing devices, etc., used in conjunction
with intracavity or in vitro ultrasound probes in ultrasound diagnosis and
treatment operations.
3.2
In-plane puncture
5.2.2 The puncture instrument shall not be obstructed during its travel in the
needle groove. And the puncture instrument must not shake significantly.
5.3 In-plane puncture accuracy
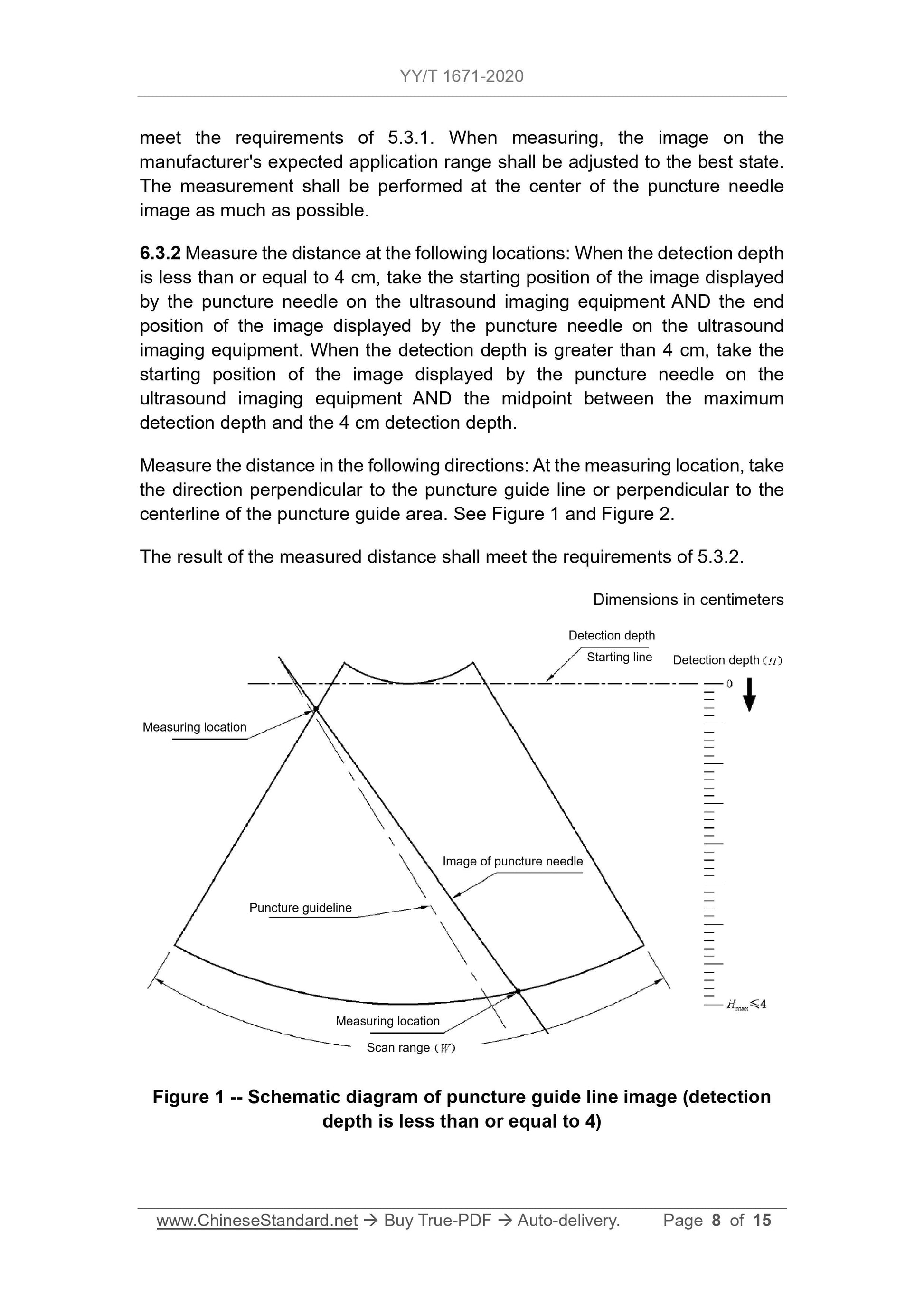
5.3.1 The puncture needle shall be clearly displayed within the image scanning
range of the ultrasound imaging equipment.
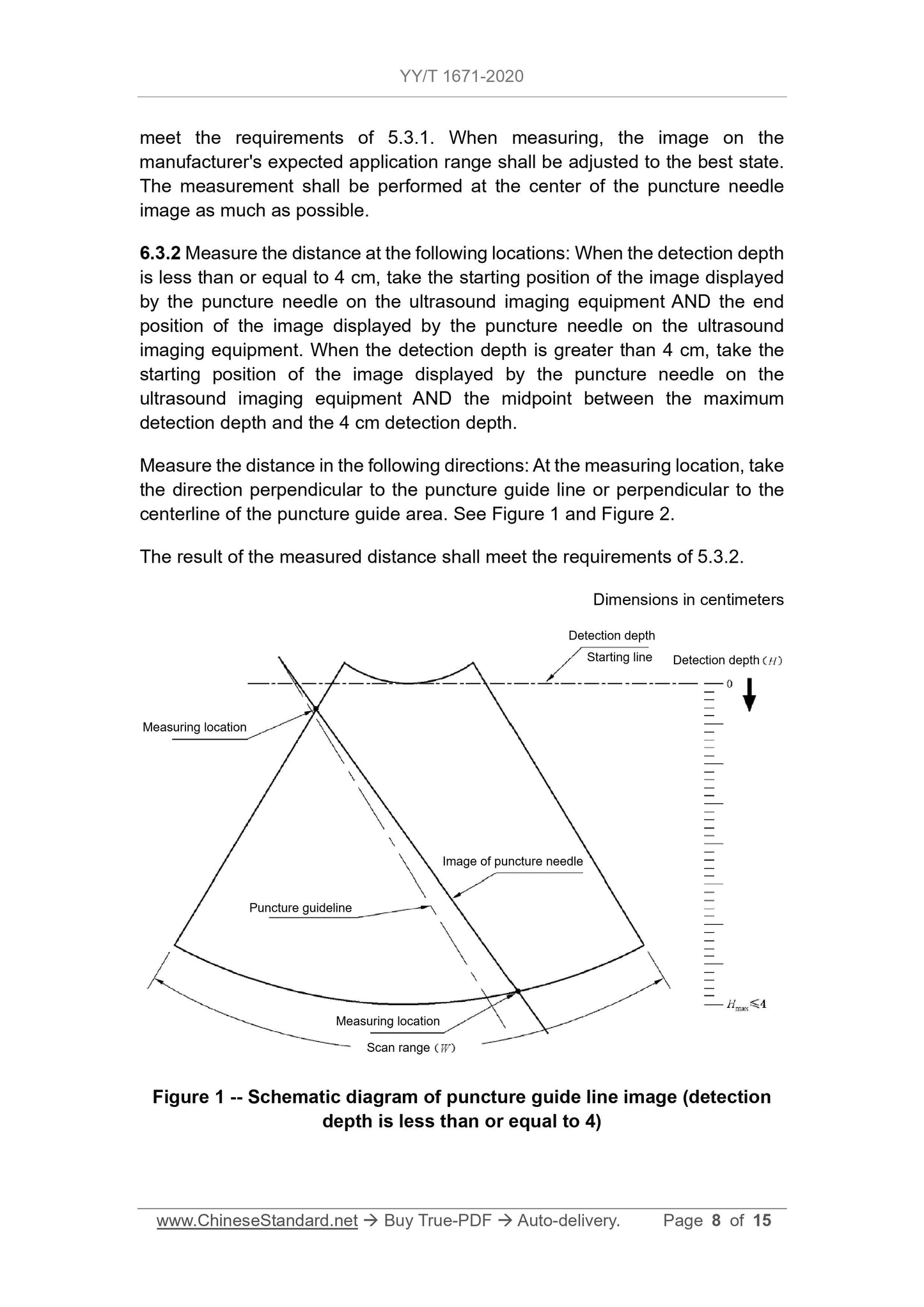
5.3.2 For ultrasound imaging equipment with puncture guide lines, when the
detection depth is less than or equal to 4 cm, the distance FROM the point on
the straight line (where the image displayed by the puncture needle on the
biopsy guide manufacturer's expected application range is located) TO the
puncture guide line shall not exceed the value specified by the biopsy guide
manufacturer. But the maximum distance must not exceed 5 mm. When the
detection depth is greater than 4 cm, the distance FROM the point on the
straight line (where the image displayed by the puncture needle on the
expected application range is located) TO the puncture guide line shall not
exceed the value specified by the biopsy guide manufacturer. But the maximum
distance must not exceed 10 mm.
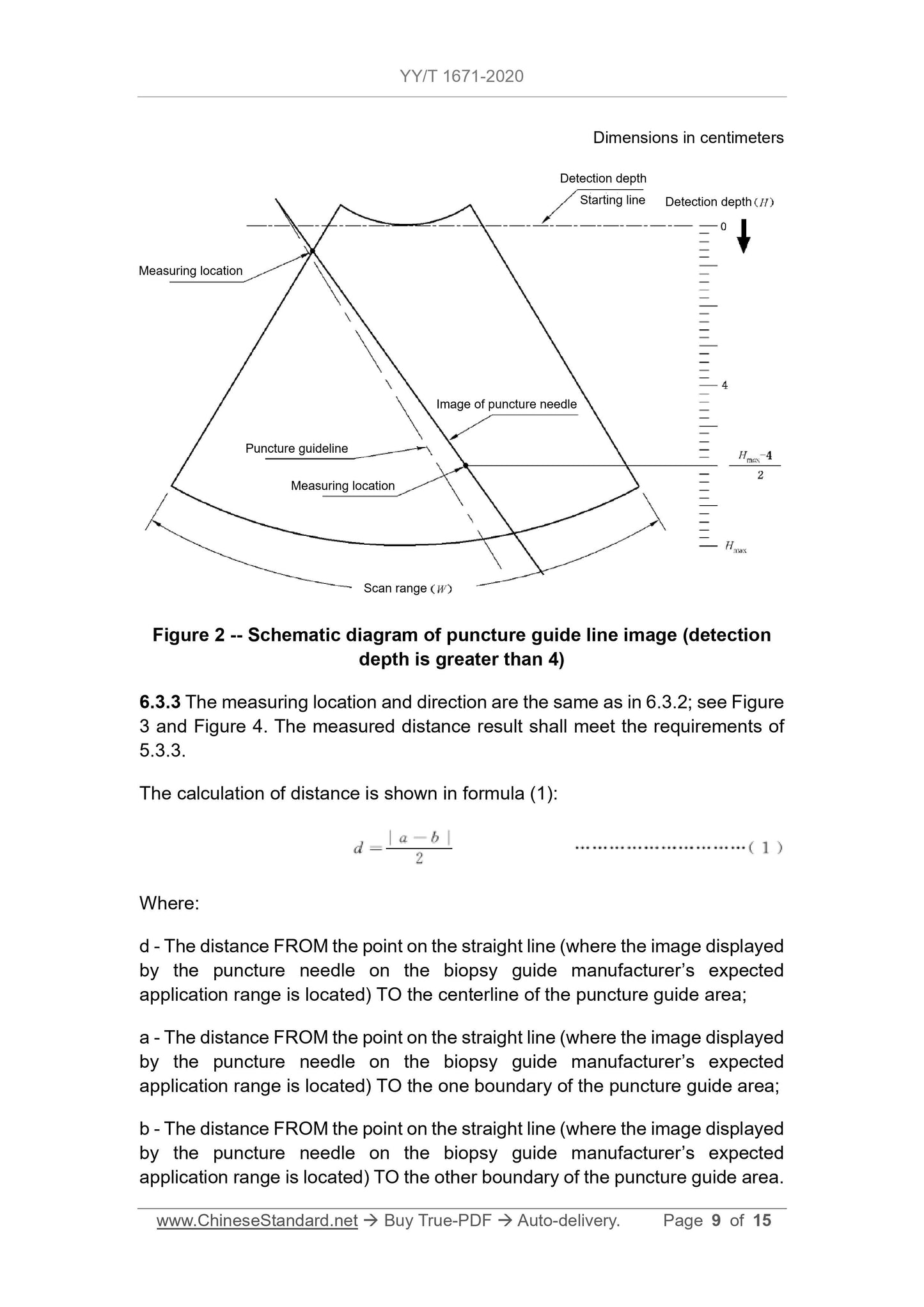
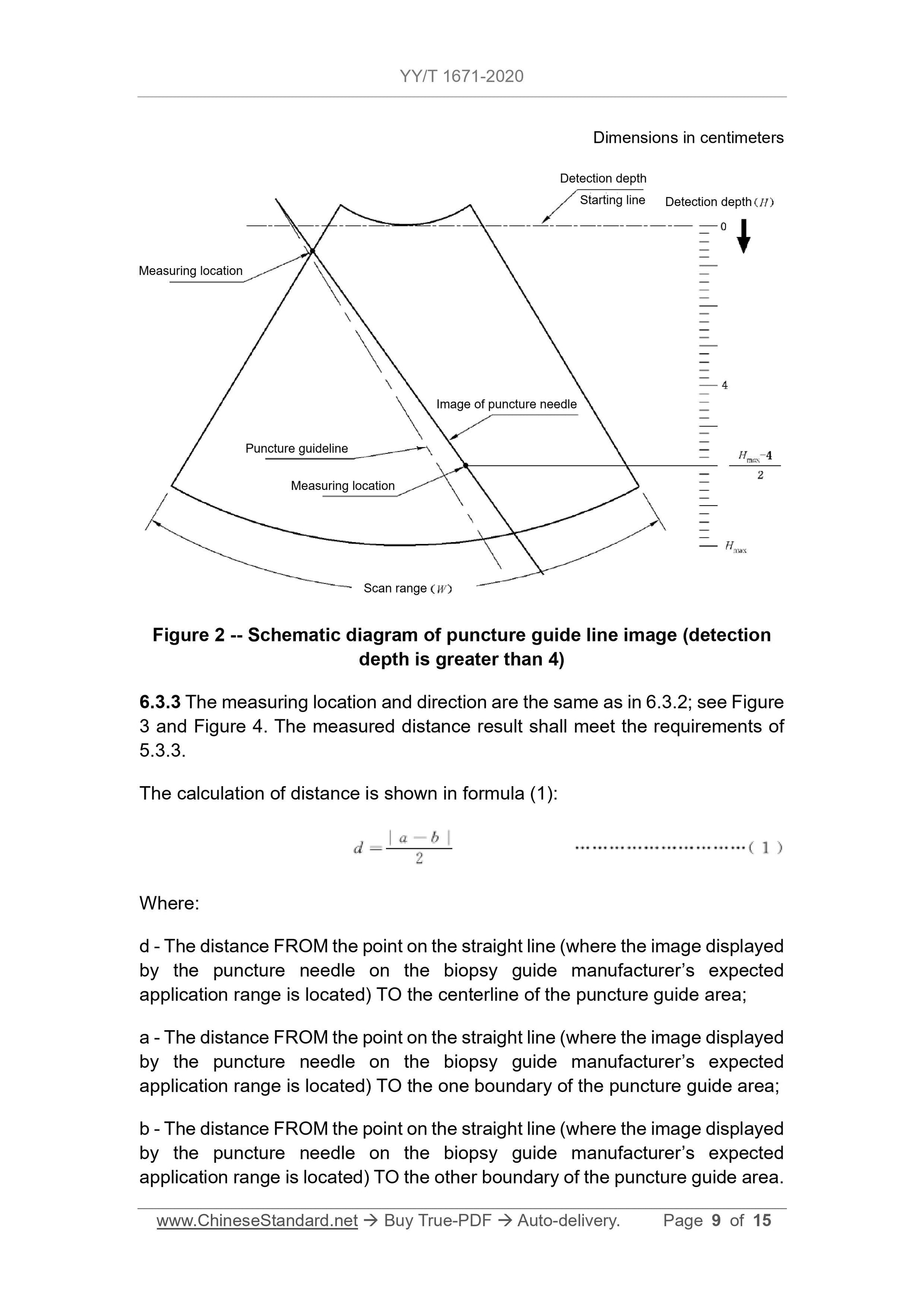
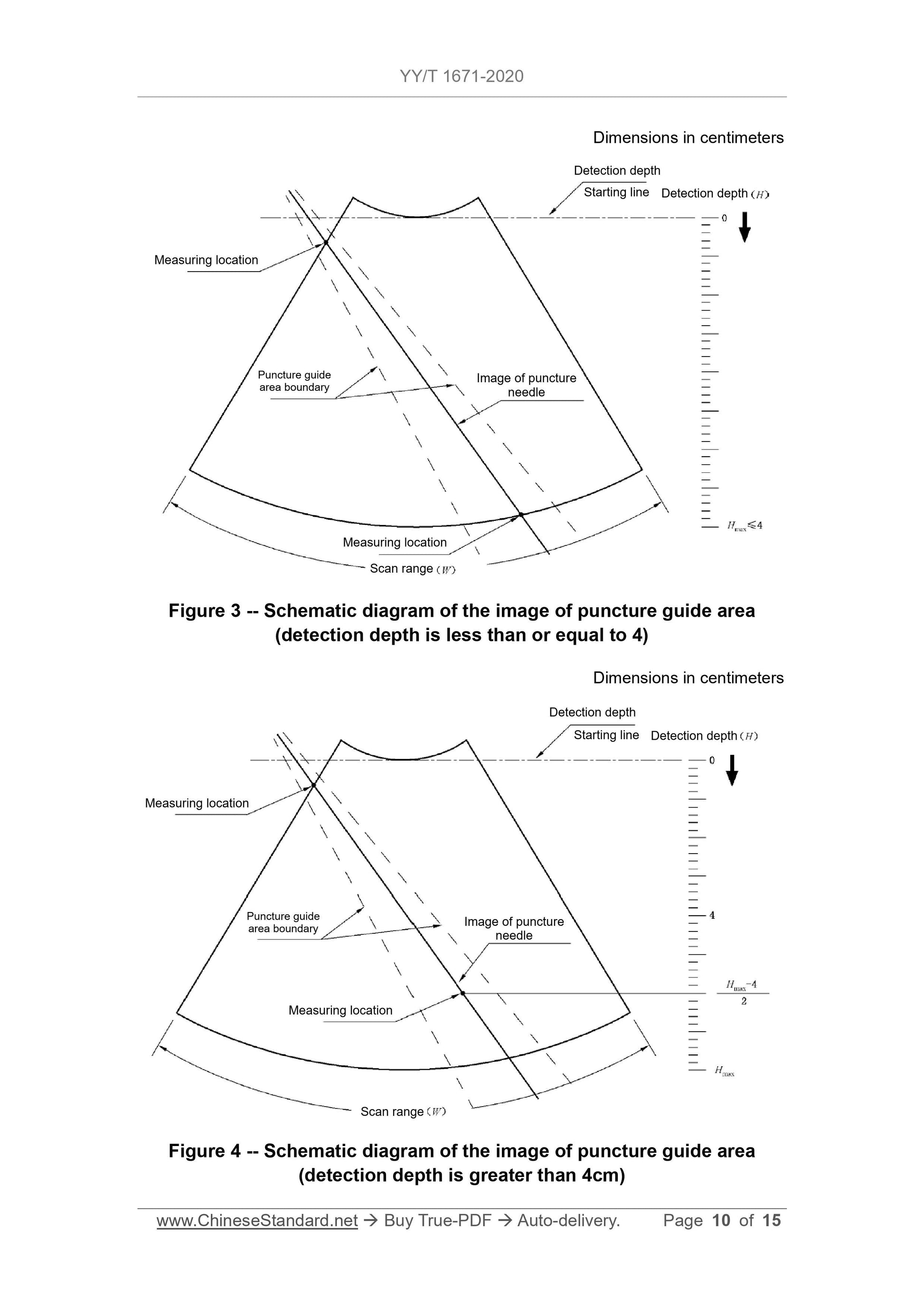
5.3.3 For ultrasound imaging equipment that only displays the puncture guide
area, when the detection depth is less than or equal to 4 cm, the distance FROM
the point on the straight line (where the image displayed by the puncture needle
on the biopsy guide manufacturer's expected application range is located) TO
the centerline of the puncture guide area shall not exceed the value specified
by the biopsy guide manufacturer. But the maximum distance must not exceed
5 mm. When the detection depth is greater than 4 cm, the distance FROM the
point on the straight line (where the image displayed by the puncture needle on
the expected application range is located) TO the centerline of the puncture
guide area shall not exceed the value specified by the biopsy guide
manufacturer. But the maximum distance must not exceed 10 mm. The image
displayed by the puncture needle on the ultrasound imaging equipment must
not exceed the scope of the puncture guide area.
5.3.4 For situations where there is no guide line or no guide line is used, the
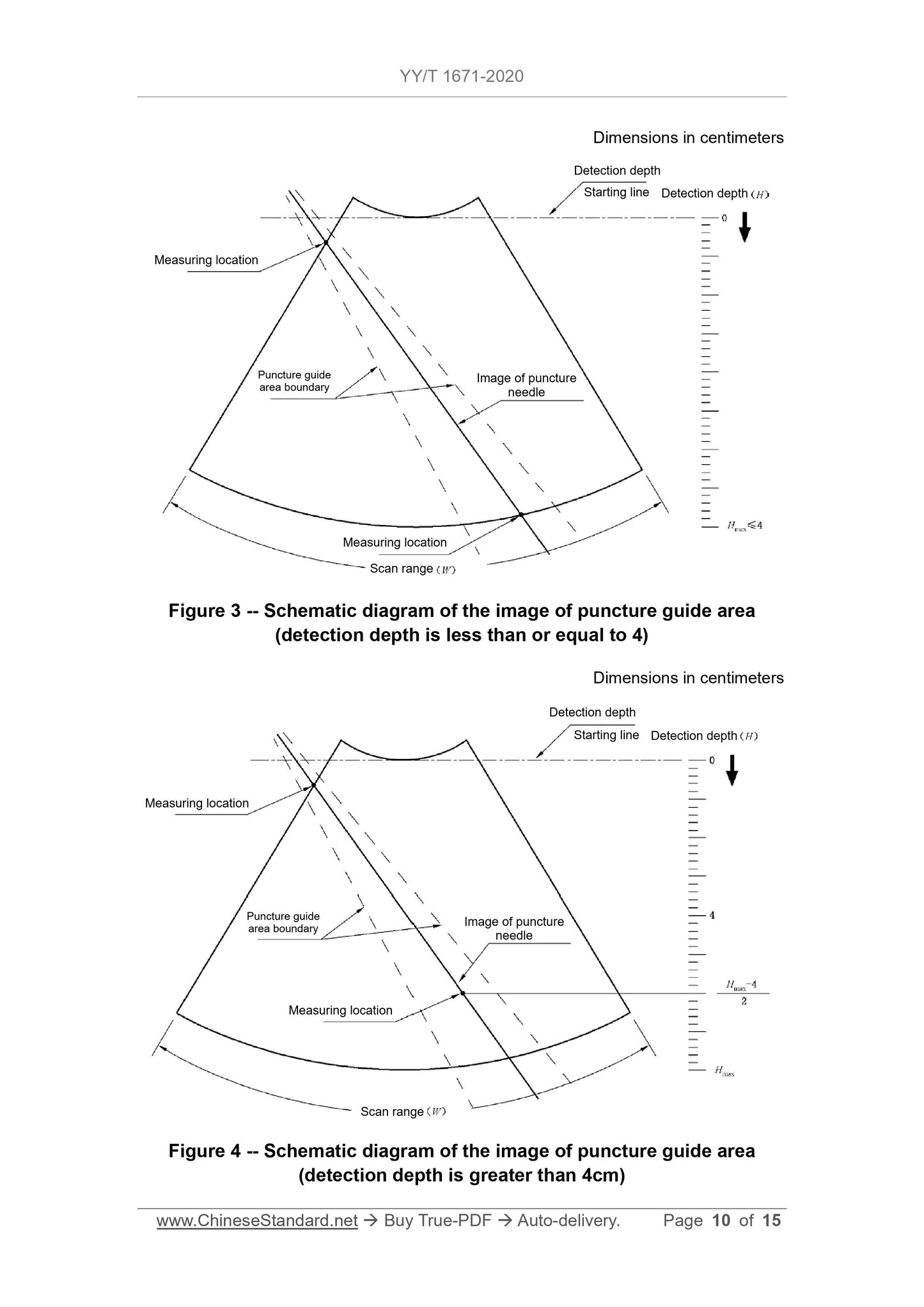
requirements of 5.3.1 shall be met.
5.3.5 For a biopsy guide equipped with multiple needle grooves, each needle
groove shall meet the requirements of 5.3.1. In addition, each needle groove
must meet the requirements of 5.3.2 or 5.3.3 or 5.3.4.
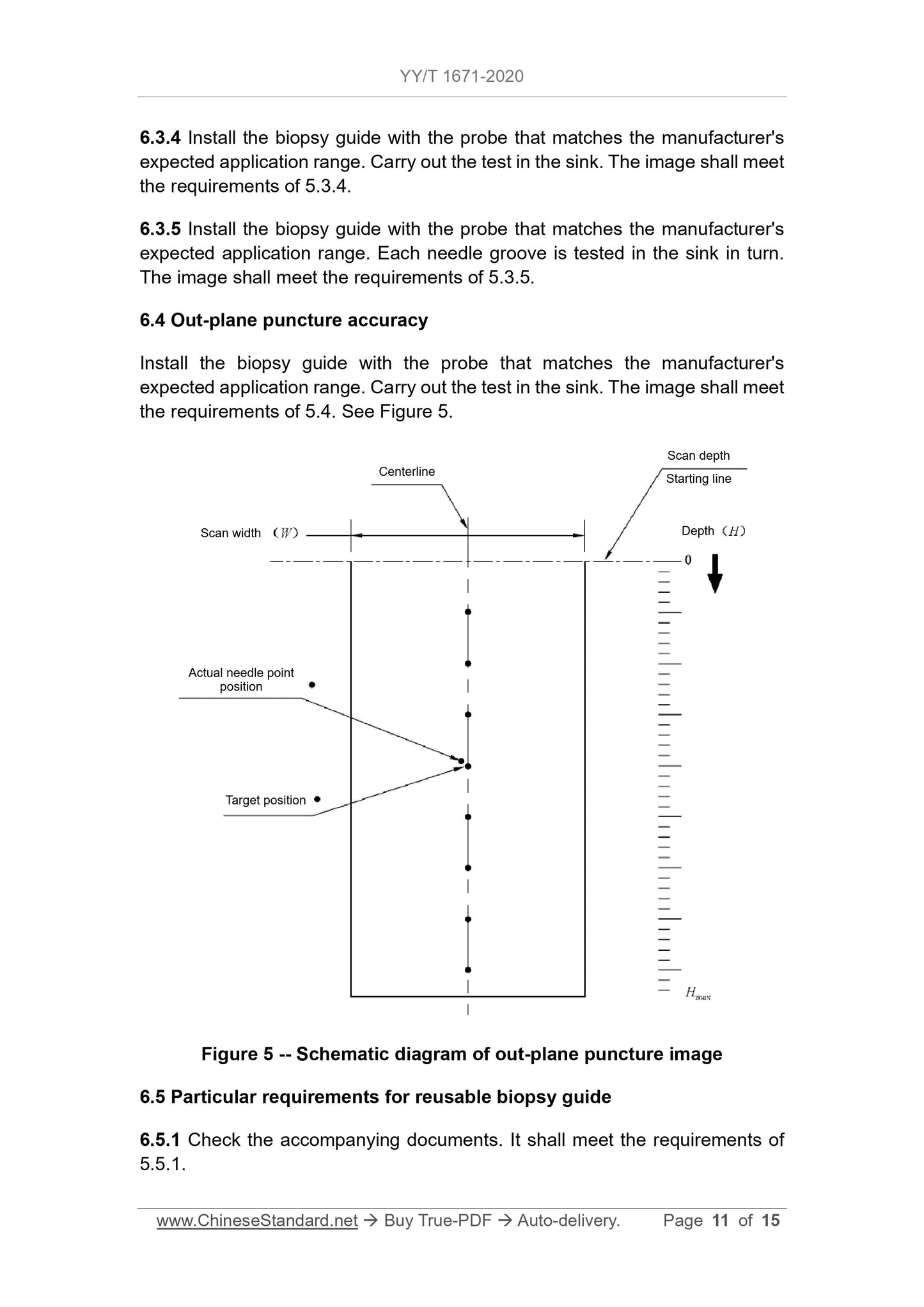
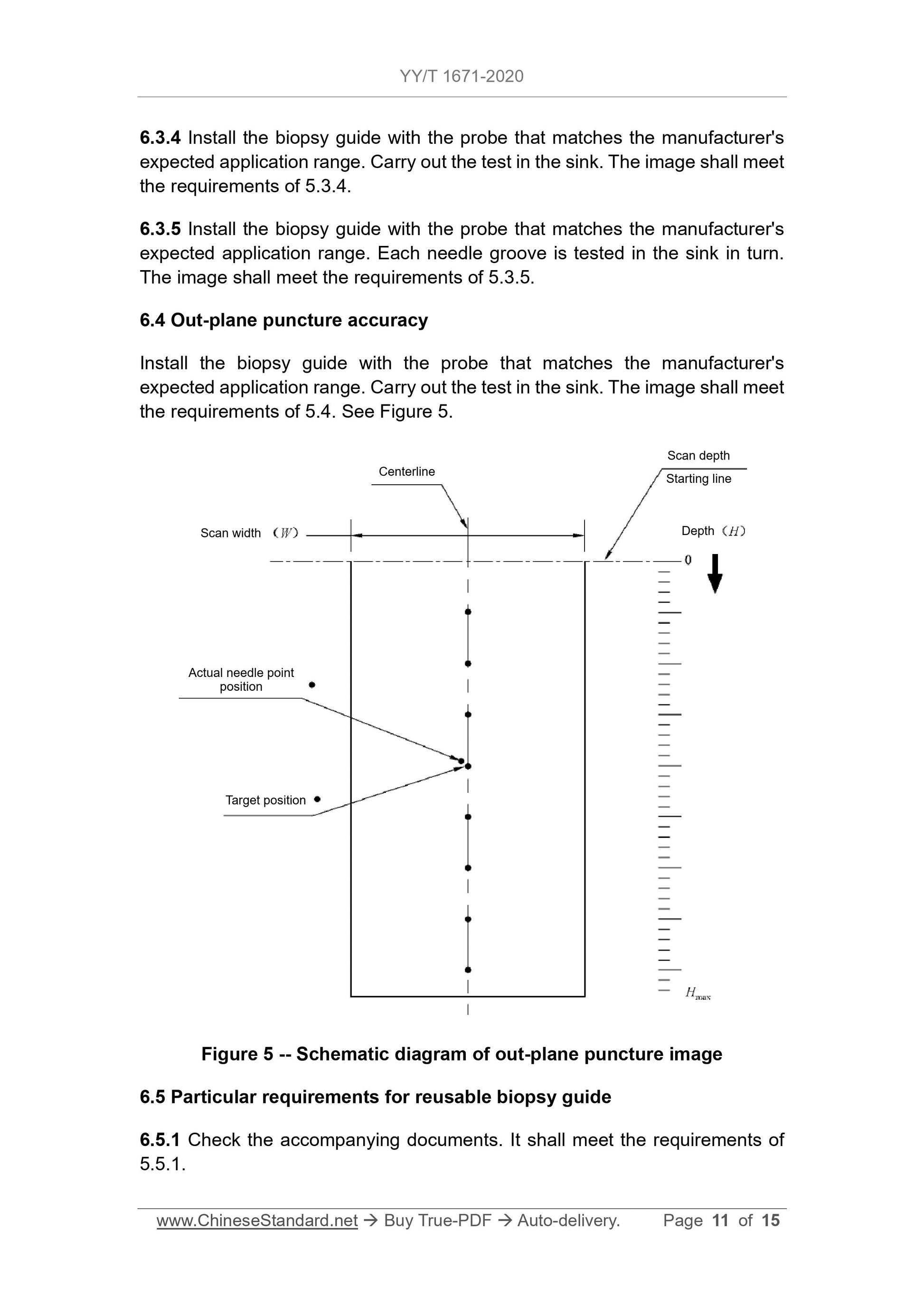
5.4 Out-plane puncture accuracy
The puncture point shall meet the depth position requirements of the biopsy
guide manufacturer's expected application range. The distance BETWEEN the
actual puncture point image of the puncture needle on the image AND the set
puncture target position shall meet the value specified by the biopsy guide
manufacturer. However, when the detection depth is less than or equal to 4 cm,
the maximum distance must not exceed 5 mm. When the detection depth is
greater than 4 cm, the maximum distance must not exceed 10 mm.
5.5 Particular requirements for reusable biopsy guide
5.5.1 In the accompanying documents, the manufacturer shall specify the
sterilization method and effective service life of the reusable biopsy guide.
5.5.2 After sterilization according to the requirements of 5.5.1, the reusable
biopsy guide can still meet the applicable performance requirements in 5.1 to
5.4.
5.6 Particular requirements for single-use biopsy guide
5.6.1 When leaving the factory, the single-use biopsy guide shall be sterile. It
shall be sterile after a confirmed sterilization process.
5.6.2 If the single-use biopsy guide is sterilized with ethylene oxide, the residual
amount of ethylene oxide shall not exceed 10 μg/g.
5.7 Biological evaluation
The biopsy guide shall be biologically evaluated in accordance with the
requirements of GB/T 16886.1. The evaluation result shall conclude free of
biological hazards.
6 Test methods
6.1 Appearance
Use visual observation or hand touching to check. It shall meet the
requirements of 5.1.
6.2 Usability
6.2.1 Use actual operations. It shall meet the requirements of 5.2.1.
6.2.2 Use actual operations. It shall meet the requirements of 5.2.2. At the same
time, its shaking must not affect the puncture accuracy.
6.3 In-plane puncture accuracy
6.3.1 Install the biopsy guide with the probe in the application range expected
by the biopsy guide manufacturer. Carry out the test in the sink. The image shall
Get QUOTATION in 1-minute: Click YY/T 1671-2020
Historical versions: YY/T 1671-2020
Preview True-PDF (Reload/Scroll if blank)
YY/T 1671-2020: Ultrasound biopsy guide
YY/T 1671-2020
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.50
C 41
Ultrasound biopsy guide
超声探头穿刺架
ISSUED ON: FEBRUARY 25, 2020
IMPLEMENTED ON: MARCH 01, 2022
Issued by: National Medical Products Administration
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative references ... 4
3 Terms and definitions ... 4
4 Structure type and nomenclature ... 5
5 Requirements... 5
6 Test methods ... 7
Appendix A (Informative) Typical types of biopsy guide structure ... 13
Ultrasound biopsy guide
1 Scope
This Standard specifies the terms and definitions, structure type and
nomenclature, requirements, and test methods for ultrasound biopsy guide.
This Standard applies to the ultrasound biopsy guide.
This Standard does not apply to inductive navigation devices, such as magnetic
navigation devices.
2 Normative references
The following documents are indispensable for the application of this document.
For the dated references, only the editions with the dates indicated are
applicable to this document. For the undated references, the latest edition
(including all the amendments) are applicable to this document.
GB/T 14233.1-2008 Test methods for infusion, transfusion, injection
equipment for medical use - Part 1: Chemical analysis methods
GB/T 16886.1 Biological evaluation of medical devices - Part 1: Evaluation
and testing within a risk management process
Pharmacopoeia of the People's Republic of China (2015)
3 Terms and definitions
The following terms and definitions apply to this document.
3.1
Ultrasound biopsy guide
A device used for guiding and fixing puncture instruments such as puncture
needles, drainage tubes, treatment or dosing devices, etc., used in conjunction
with intracavity or in vitro ultrasound probes in ultrasound diagnosis and
treatment operations.
3.2
In-plane puncture
5.2.2 The puncture instrument shall not be obstructed during its travel in the
needle groove. And the puncture instrument must not shake significantly.
5.3 In-plane puncture accuracy
5.3.1 The puncture needle shall be clearly displayed within the image scanning
range of the ultrasound imaging equipment.
5.3.2 For ultrasound imaging equipment with puncture guide lines, when the
detection depth is less than or equal to 4 cm, the distance FROM the point on
the straight line (where the image displayed by the puncture needle on the
biopsy guide manufacturer's expected application range is located) TO the
puncture guide line shall not exceed the value specified by the biopsy guide
manufacturer. But the maximum distance must not exceed 5 mm. When the
detection depth is greater than 4 cm, the distance FROM the point on the
straight line (where the image displayed by the puncture needle on the
expected application range is located) TO the puncture guide line shall not
exceed the value specified by the biopsy guide manufacturer. But the maximum
distance must not exceed 10 mm.
5.3.3 For ultrasound imaging equipment that only displays the puncture guide
area, when the detection depth is less than or equal to 4 cm, the distance FROM
the point on the straight line (where the image displayed by the puncture needle
on the biopsy guide manufacturer's expected application range is located) TO
the centerline of the puncture guide area shall not exceed the value specified
by the biopsy guide manufacturer. But the maximum distance must not exceed
5 mm. When the detection depth is greater than 4 cm, the distance FROM the
point on the straight line (where the image displayed by the puncture needle on
the expected application range is located) TO the centerline of the puncture
guide area shall not exceed the value specified by the biopsy guide
manufacturer. But the maximum distance must not exceed 10 mm. The image
displayed by the puncture needle on the ultrasound imaging equipment must
not exceed the scope of the puncture guide area.
5.3.4 For situations where there is no guide line or no guide line is used, the
requirements of 5.3.1 shall be met.
5.3.5 For a biopsy guide equipped with multiple needle grooves, each needle
groove shall meet the requirements of 5.3.1. In addition, each needle groove
must meet the requirements of 5.3.2 or 5.3.3 or 5.3.4.
5.4 Out-plane puncture accuracy
The puncture point shall meet the depth position requirements of the biopsy
guide manufacturer's expected application range. The distance BETWEEN the
actual puncture point image of the puncture needle on the image AND the set
puncture target position shall meet the value specified by the biopsy guide
manufacturer. However, when the detection depth is less than or equal to 4 cm,
the maximum distance must not exceed 5 mm. When the detection depth is
greater than 4 cm, the maximum distance must not exceed 10 mm.
5.5 Particular requirements for reusable biopsy guide
5.5.1 In the accompanying documents, the manufacturer shall specify the
sterilization method and effective service life of the reusable biopsy guide.
5.5.2 After sterilization according to the requirements of 5.5.1, the reusable
biopsy guide can still meet the applicable performance requirements in 5.1 to
5.4.
5.6 Particular requirements for single-use biopsy guide
5.6.1 When leaving the factory, the single-use biopsy guide shall be sterile. It
shall be sterile after a confirmed sterilization process.
5.6.2 If the single-use biopsy guide is sterilized with ethylene oxide, the residual
amount of ethylene oxide shall not exceed 10 μg/g.
5.7 Biological evaluation
The biopsy guide shall be biologically evaluated in accordance with the
requirements of GB/T 16886.1. The evaluation result shall conclude free of
biological hazards.
6 Test methods
6.1 Appearance
Use visual observation or hand touching to check. It shall meet the
requirements of 5.1.
6.2 Usability
6.2.1 Use actual operations. It shall meet the requirements of 5.2.1.
6.2.2 Use actual operations. It shall meet the requirements of 5.2.2. At the same
time, its shaking must not affect the puncture accuracy.
6.3 In-plane puncture accuracy
6.3.1 Install the biopsy guide with the probe in the application range expected
by the biopsy guide manufacturer. Carry out the test in the sink. The image shall
Share