1
/
の
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1302.2-2015 English PDF (YYT1302.2-2015)
YY/T 1302.2-2015 English PDF (YYT1302.2-2015)
通常価格
$150.00 USD
通常価格
セール価格
$150.00 USD
単価
/
あたり
配送料はチェックアウト時に計算されます。
受取状況を読み込めませんでした
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY/T 1302.2-2015
Historical versions: YY/T 1302.2-2015
Preview True-PDF (Reload/Scroll if blank)
YY/T 1302.2-2015: Physical requirements and microbiological performance of ethylene oxide sterilization. Part 2: Microbiological aspects
YY/T 1302.2-2015
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.080.01
C 47
Physical requirements and microbiological
performance of ethylene oxide sterilization -
Part 2: Microbiological aspects
ISSUED ON: MARCH 02, 2015
IMPLEMENTED ON: JANUARY 01, 2016
Issued by: China Food and Drug Administration
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative references ... 4
3 Terms and definitions ... 5
4 Process definition ... 5
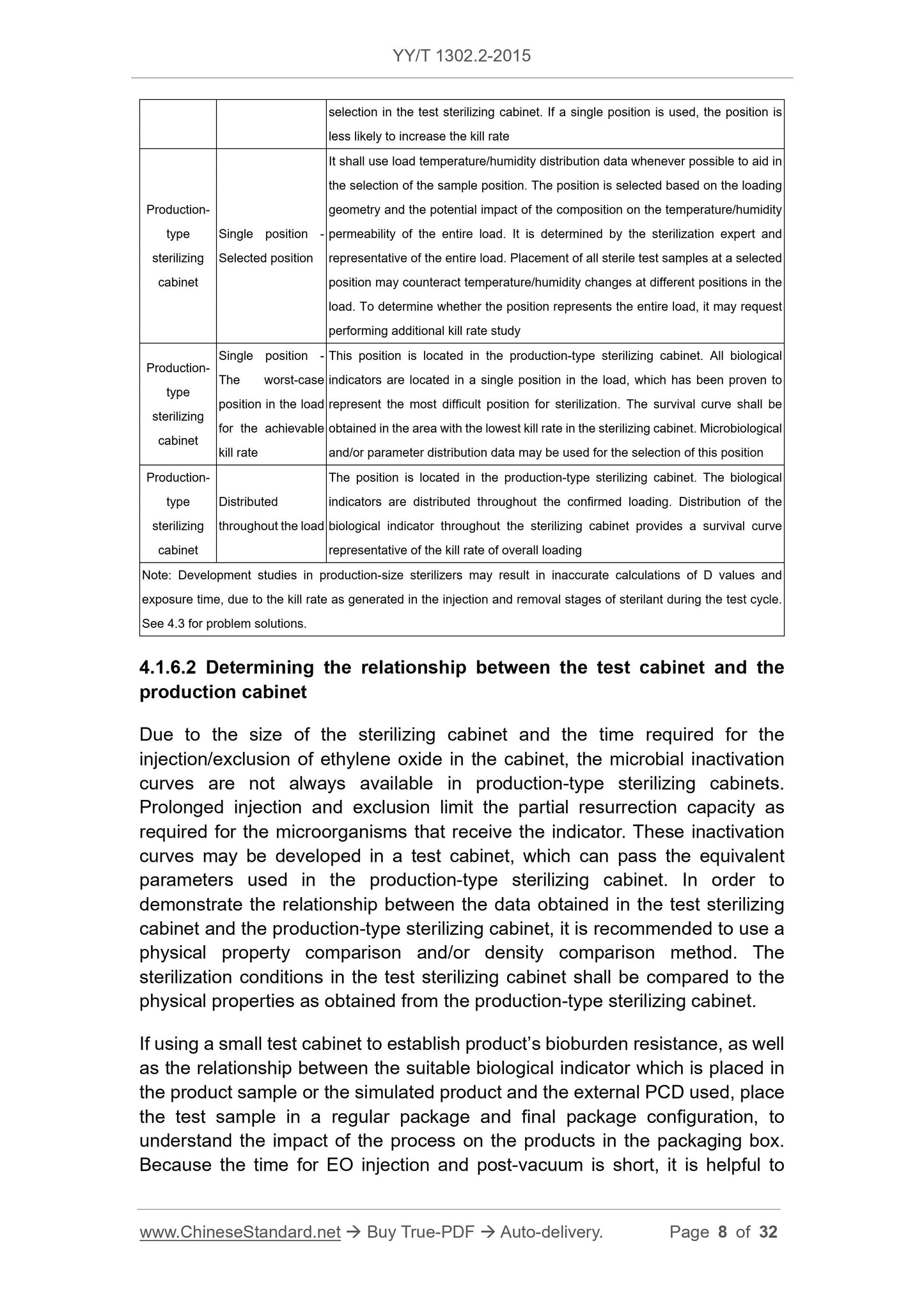
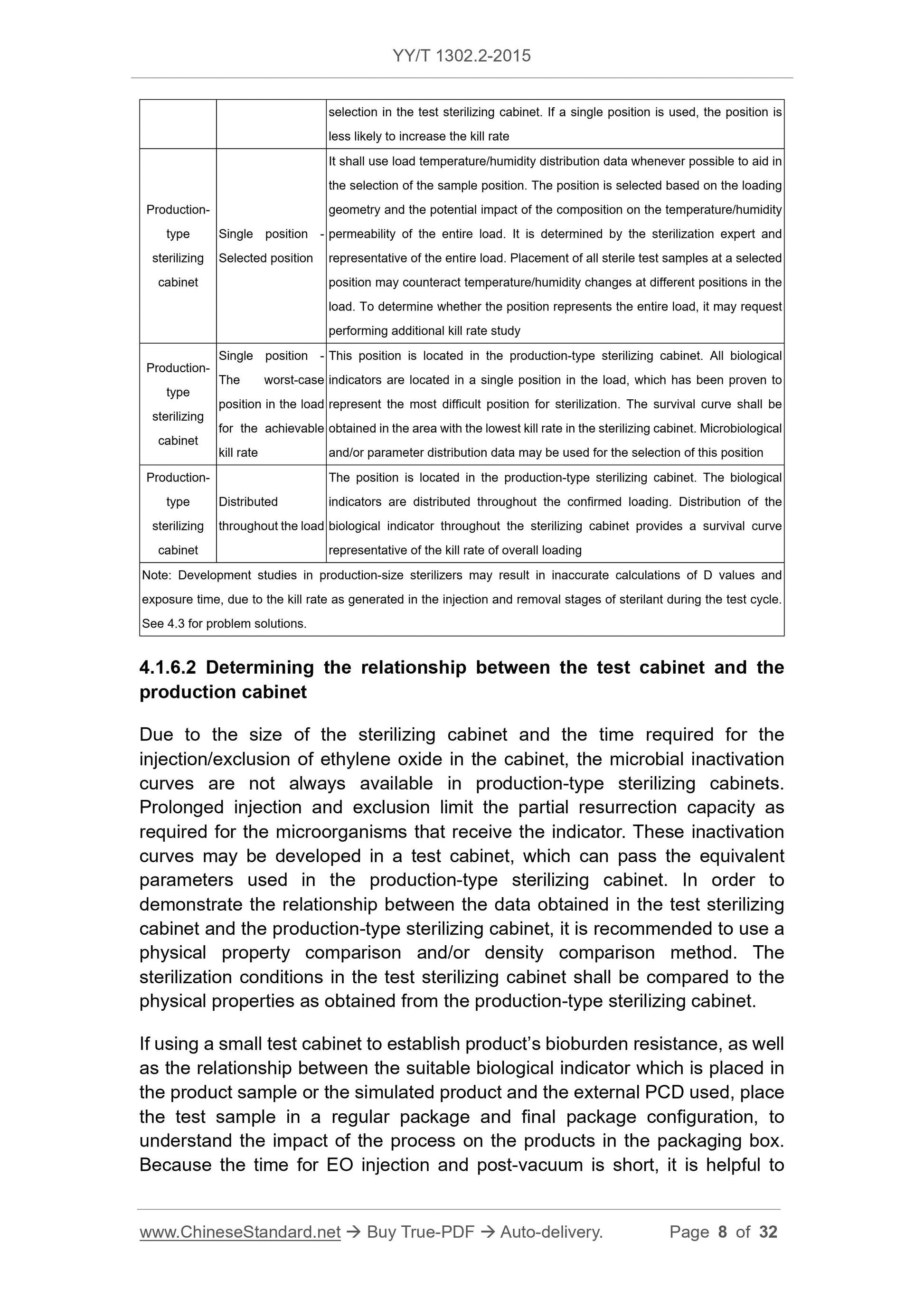
4.1 Considerations of process definition ... 5
4.2 Process definition method ... 10
4.3 Troubleshooting of the sterilization process definition ... 17
4.4 Process challenge device (PCD) ... 19
5 Validation ... 23
5.1 Microbial performance qualification (MPQ) ... 23
5.2 Sterilization loading ... 25
5.3 Simulating expected process conditions ... 27
5.4 Confirming the release of load ... 27
5.5 Small batch release ... 28
6 Maintenance of process effectiveness ... 29
6.1 Failure investigation ... 29
6.2 Re-qualification ... 31
Physical requirements and microbiological
performance of ethylene oxide sterilization -
Part 2: Microbiological aspects
1 Scope
This part of YY/T 1302 specifies process definition, validation, process
effectiveness maintenance, etc. for the microbiological aspects of ethylene
oxide sterilization.
This part applies to the ethylene oxide sterilization process for medical devices
and other related products or materials, provides solutions to various
microbiological aspects in the development and validation of ethylene oxide
(EO) sterilization processes. This part also provides additional application
guidelines for medical device manufacturers that use the ISO 11135-1:2007 and
ISO/TS 11135-2:2008 standards, including those that use outsourced
sterilization plants or outsourced sterilization operations.
This part of YY/T 1302 does not include various factors that may affect the
product's bioburden and sterilization process.
2 Normative references
The following documents are essential to the application of this document. For
the dated documents, only the versions with the dates indicated are applicable
to this document; for the undated documents, only the latest version (including
all the amendments) are applicable to this standard.
GB/T 19974-2005 Sterilization of health care products - General requirement
for characterization of a sterilization agent and the development, validation
and routine control of a sterilization process for medical devices
ISO 11135-1:2007 Sterilization of health care products - Ethylene oxide -
Part 1: Requirements for development, validation and routine control of a
sterilization process of medical devices
ISO/TS 11135-2:2008 Sterilization of health care products - Ethylene oxide -
Part 2: Guidance on the application of ISO 11135-1
ISO 11138-1:2006 Sterilization of health care products - Biological indicators
development method is based on a number of factors, including the nature of
the product's bioburden, packaging, production conditions, sterilization
equipment, cost. Usually the biological indicator (BI) / bioburden (overkill) or
other established methods are used to develop the parameters required to
achieve the sterility assurance level (SAL) as required by the product.
4.1.2 Parameters on ethylene oxide exposure
Use the cycle development information and consider the SAL of the involved
products to calculate the parameters of cyclic exposure of ethylene oxide. The
recognized SAL includes:
a) For the product that contacts compromised tissue or a sterile part of the
body, the SAL is 10-6;
b) For the product that does not contact the compromised tissue or a sterile
part of the body, the SAL is 10-3.
Note: The SAL for products labeled “sterile” is usually 10-6. SAL requirements for
products labeled as “sterile” may vary from country to country.
Products with multiple sterility assurance levels - Some products contain
components or assemblies that are different for the intended use. In the kit,
components for un-compromised skin or mucosa or that are not expected to
come into contact with the patient have different SAL requirements than
components that are expected to come into contact with internal tissues,
nervous systems or blood. Based on the intended use of the device, the
sterilization process shall achieve the required kill rate for each component.
4.1.3 Product packaging
The product package shall be breathable and resistant to changes of
vacuum/pressure rise as well as vacuum/pressure rise rates.
4.1.4 Process development method
If the product is produced in a controlled environment and the amount of
bioburden continues to be low, the process development method used to
identify sterilization parameters may be the bioburden/biological indicator
method. But it needs understanding different microbial species on the product.
4.1.5 Considerations in sampling for process development study
Before the start of the study, the process development study shall consider the
following two factors:
a) Determine the method of use: Partial negative method or direct counting
method. If partial negative method is used to obtain data, it is
compare the suitability of BI/IPCD with the EPCD to be used for routine
monitoring. Since the multi-layer penetration of the loading of conventional
product pallet will affect the final kill rate, it takes longer to achieve the same kill
rate as obtained in the test cabinet. Therefore, all parameters developed in the
test sterilizing cabinet shall be determined in the production-type equipment as
a part of the validation process.
In addition, the ratio of the test-type load volume to the available volume of the
test-type cabinet shall represent the ratio of the load volume used in the
production-type cabinet to the volume of production-type cabinet. The
comparison between test-type load and the production-type load shall be based
on the equivalence of the load, which shall be equivalent not only in terms of
weight/volume ratio, but also in terms of the challenge of the product and the
loading configuration for the sterilization process.
4.1.6.3 Parameters
It may compare the following factors to determine the relationship between the
studies as performed in both the test-type and production-type sterilizing
cabinets:
a) Setting value and range of temperature inside the preconditioning room (if
used);
b) Setting value and range of relative humidity inside the preconditioning
room (if used);
c) Preconditioning time;
d) Setting value and range of temperature in the sterilizing cabinet;
e) Setting value and range of relative humidity in the sterilizing cabinet;
f) Setting value and range of gas concentration in the sterilizing cabinet (if
there is a gas analysis instrument in the test sterilizing cabinet);
g) The sterilant (gas mixture) used (i.e., the volume fraction of all gases);
h) Gas residence time;
i) Vacuum pressure / transfer depth and rate;
j) Microbial kill rate;
k) Setting value and range of temperature in the analysis room (if used);
l) Range of temperature and relative humidity in the load.
It is generally considered that the position of lowest temperature or the position
Note: At the end of the cycle, it shall retrieve the BI and product sample as soon as
possible. Once the sample is retrieved, follow the confirmed method to carry out the
biological test.
4.2...
Get QUOTATION in 1-minute: Click YY/T 1302.2-2015
Historical versions: YY/T 1302.2-2015
Preview True-PDF (Reload/Scroll if blank)
YY/T 1302.2-2015: Physical requirements and microbiological performance of ethylene oxide sterilization. Part 2: Microbiological aspects
YY/T 1302.2-2015
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.080.01
C 47
Physical requirements and microbiological
performance of ethylene oxide sterilization -
Part 2: Microbiological aspects
ISSUED ON: MARCH 02, 2015
IMPLEMENTED ON: JANUARY 01, 2016
Issued by: China Food and Drug Administration
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative references ... 4
3 Terms and definitions ... 5
4 Process definition ... 5
4.1 Considerations of process definition ... 5
4.2 Process definition method ... 10
4.3 Troubleshooting of the sterilization process definition ... 17
4.4 Process challenge device (PCD) ... 19
5 Validation ... 23
5.1 Microbial performance qualification (MPQ) ... 23
5.2 Sterilization loading ... 25
5.3 Simulating expected process conditions ... 27
5.4 Confirming the release of load ... 27
5.5 Small batch release ... 28
6 Maintenance of process effectiveness ... 29
6.1 Failure investigation ... 29
6.2 Re-qualification ... 31
Physical requirements and microbiological
performance of ethylene oxide sterilization -
Part 2: Microbiological aspects
1 Scope
This part of YY/T 1302 specifies process definition, validation, process
effectiveness maintenance, etc. for the microbiological aspects of ethylene
oxide sterilization.
This part applies to the ethylene oxide sterilization process for medical devices
and other related products or materials, provides solutions to various
microbiological aspects in the development and validation of ethylene oxide
(EO) sterilization processes. This part also provides additional application
guidelines for medical device manufacturers that use the ISO 11135-1:2007 and
ISO/TS 11135-2:2008 standards, including those that use outsourced
sterilization plants or outsourced sterilization operations.
This part of YY/T 1302 does not include various factors that may affect the
product's bioburden and sterilization process.
2 Normative references
The following documents are essential to the application of this document. For
the dated documents, only the versions with the dates indicated are applicable
to this document; for the undated documents, only the latest version (including
all the amendments) are applicable to this standard.
GB/T 19974-2005 Sterilization of health care products - General requirement
for characterization of a sterilization agent and the development, validation
and routine control of a sterilization process for medical devices
ISO 11135-1:2007 Sterilization of health care products - Ethylene oxide -
Part 1: Requirements for development, validation and routine control of a
sterilization process of medical devices
ISO/TS 11135-2:2008 Sterilization of health care products - Ethylene oxide -
Part 2: Guidance on the application of ISO 11135-1
ISO 11138-1:2006 Sterilization of health care products - Biological indicators
development method is based on a number of factors, including the nature of
the product's bioburden, packaging, production conditions, sterilization
equipment, cost. Usually the biological indicator (BI) / bioburden (overkill) or
other established methods are used to develop the parameters required to
achieve the sterility assurance level (SAL) as required by the product.
4.1.2 Parameters on ethylene oxide exposure
Use the cycle development information and consider the SAL of the involved
products to calculate the parameters of cyclic exposure of ethylene oxide. The
recognized SAL includes:
a) For the product that contacts compromised tissue or a sterile part of the
body, the SAL is 10-6;
b) For the product that does not contact the compromised tissue or a sterile
part of the body, the SAL is 10-3.
Note: The SAL for products labeled “sterile” is usually 10-6. SAL requirements for
products labeled as “sterile” may vary from country to country.
Products with multiple sterility assurance levels - Some products contain
components or assemblies that are different for the intended use. In the kit,
components for un-compromised skin or mucosa or that are not expected to
come into contact with the patient have different SAL requirements than
components that are expected to come into contact with internal tissues,
nervous systems or blood. Based on the intended use of the device, the
sterilization process shall achieve the required kill rate for each component.
4.1.3 Product packaging
The product package shall be breathable and resistant to changes of
vacuum/pressure rise as well as vacuum/pressure rise rates.
4.1.4 Process development method
If the product is produced in a controlled environment and the amount of
bioburden continues to be low, the process development method used to
identify sterilization parameters may be the bioburden/biological indicator
method. But it needs understanding different microbial species on the product.
4.1.5 Considerations in sampling for process development study
Before the start of the study, the process development study shall consider the
following two factors:
a) Determine the method of use: Partial negative method or direct counting
method. If partial negative method is used to obtain data, it is
compare the suitability of BI/IPCD with the EPCD to be used for routine
monitoring. Since the multi-layer penetration of the loading of conventional
product pallet will affect the final kill rate, it takes longer to achieve the same kill
rate as obtained in the test cabinet. Therefore, all parameters developed in the
test sterilizing cabinet shall be determined in the production-type equipment as
a part of the validation process.
In addition, the ratio of the test-type load volume to the available volume of the
test-type cabinet shall represent the ratio of the load volume used in the
production-type cabinet to the volume of production-type cabinet. The
comparison between test-type load and the production-type load shall be based
on the equivalence of the load, which shall be equivalent not only in terms of
weight/volume ratio, but also in terms of the challenge of the product and the
loading configuration for the sterilization process.
4.1.6.3 Parameters
It may compare the following factors to determine the relationship between the
studies as performed in both the test-type and production-type sterilizing
cabinets:
a) Setting value and range of temperature inside the preconditioning room (if
used);
b) Setting value and range of relative humidity inside the preconditioning
room (if used);
c) Preconditioning time;
d) Setting value and range of temperature in the sterilizing cabinet;
e) Setting value and range of relative humidity in the sterilizing cabinet;
f) Setting value and range of gas concentration in the sterilizing cabinet (if
there is a gas analysis instrument in the test sterilizing cabinet);
g) The sterilant (gas mixture) used (i.e., the volume fraction of all gases);
h) Gas residence time;
i) Vacuum pressure / transfer depth and rate;
j) Microbial kill rate;
k) Setting value and range of temperature in the analysis room (if used);
l) Range of temperature and relative humidity in the load.
It is generally considered that the position of lowest temperature or the position
Note: At the end of the cycle, it shall retrieve the BI and product sample as soon as
possible. Once the sample is retrieved, follow the confirmed method to carry out the
biological test.
4.2...
Share