1
/
の
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1571-2017 English PDF (YYT1571-2017)
YY/T 1571-2017 English PDF (YYT1571-2017)
通常価格
$320.00 USD
通常価格
セール価格
$320.00 USD
単価
/
あたり
配送料はチェックアウト時に計算されます。
受取状況を読み込めませんでした
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY/T 1571-2017
Historical versions: YY/T 1571-2017
Preview True-PDF (Reload/Scroll if blank)
YY/T 1571-2017: Tissue engineering medical device products—Sodium hyaluronate
YY/T 1571-2017
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.40
C 45
Replacing YY/T 0606.9-2007
Tissue engineering medical device products - Sodium
hyaluronate
组织工程医疗器械产品 透明质酸钠
ISSUED ON: MAY 02, 2017
IMPLEMENTED ON: APRIL 01, 2018
Issued by: China Food and Drug Administration
Table of Contents
Foreword ... 3
1 Scope ... 6
2 Normative references ... 6
3 Terms and definitions ... 7
4 Classification ... 7
5 Requirements ... 8
6 Test methods ... 10
7 Marking ... 14
8 Packaging, transportation and storage ... 15
Appendix A (Normative) Determination of sodium hyaluronate content ... 16
Appendix B (Normative) Determination of protein content ... 19
Appendix C (Normative) Determination of residual ethanol (headspace gas
chromatography) ... 21
Appendix D (Informative) Determination of residual quaternary ammonium salt
(cetylpyridinium chloride) ... 23
Appendix E (Normative) Determination of weight-average molecular weight and
molecular weight distribution coefficient ... 26
References ... 28
Tissue engineering medical device products - Sodium
hyaluronate
1 Scope
This Standard specifies the requirements and test methods for sodium hyaluronate used
in surgical implants and tissue engineering medical device products.
This Standard applies to the preparation of sodium hyaluronate for tissue engineering
medical device products and their scaffold materials.
2 Normative references
The following referenced documents are indispensable for the application of this
document. For dated references, only the edition cited applies to this document. For
undated references, the latest edition (including any amendment) applies to this
document.
GB/T 191, Packaging - Pictorial marking for handling of goods
GB/T 14518, Determination of the pH of adhesives
GB/T 16886.1, Biological evaluation of medical devices - Part 1: Evaluation and
testing within a risk management process (GB/T 16886.1-2011, ISO 10993-1:2009,
IDT)
GB 18278 (all parts), Sterilization of health care products - Moist heat
GB 18279 (all parts), Sterilization of health care products - Ethylene oxide
GB 18280 (all parts), Sterilization of health care products - Radiation
YY/T 0313, Medical polymer products - Requirement for package and information
supplied by manufacturer
YY/T 0606.25, Tissue engineered medical product - Part 25: Quantification of
remnant DNA in biological materials utilizing animal tissues and their derivatives:
Fluorescence method
YY/T 0771.1, Medical devices utilizing animal tissues and their derivatives - Part 1:
Application of risk management (YY/T 0771.1-2009, ISO 22442-1:2007, IDT)
5 Requirements
5.1 Appearance
White or off-white powder or granular or fibrous solid, without any visible foreign
matter.
5.2 Identification
Typical Fourier transform infrared spectrum (FT-IR) frequencies (cm-1) of sodium
hyaluronate are 3 275 ~ 3 390(b), 1 615(s), 1 405(m), 1 377(m), 1 150, 1 077, 1 045(s),
946(m), 893(w). The wavenumber error of the measured band in the fingerprint area
shall be less than the specified value ±5 cm-1 (0.5%).
Note: s, strong; m, medium; w, weak; b, broad.
5.3 Sodium hyaluronate content
Based on the dry product, the content of sodium hyaluronate shall be 95.0% ~ 105.0%
(mass fraction).
5.4 pH
The pH of the 0.5% concentration solution shall be 5.0 ~ 8.5.
5.5 Intrinsic viscosity
It shall be 90% ~ 120% of its marked value.
5.6 Protein content
It shall not exceed 0.1% (mass fraction).
5.7 Nucleic acid content
5.7.1 Bacterial fermentation method
For solution of the concentration 0.33%, the absorbance value (OD value) at the
wavelength of 260 nm shall not exceed 0.5.
5.7.2 Tissue extraction method
In accordance with YY/T 0606.25, the limit value of residual DNA is given according
to the possible intended use.
5.8 Heavy metal content
It shall not exceed 10 μg/g (mass fraction).
The number of bacterial colonies per 1 g of the test product shall not exceed 102 CFU;
the number of mold and yeast colonies shall not exceed 20 CFU. Staphylococcus aureus,
Pseudomonas aeruginosa and Escherichia coli shall not be detected.
Note: If sodium hyaluronate is provided in a non-sterile manner, this item shall be tested;
if the final product is required to be sterile, it should be sterilized to meet the
sterile requirements.
5.19 Bacterial endotoxins
The amount of endotoxin contained per 1 mg of sodium hyaluronate shall be less than
0.05 EU.
5.20 Raw material safety
5.20.1 The sodium hyaluronate prepared by the bacterial fermentation method shall be
tested for hemolytic streptolysin, and the result shall be free from hemolytic ring.
5.20.2 The sodium hyaluronate prepared by the tissue extraction method shall be
subjected to safety evaluation and quality control in accordance with the requirements
of the series industry standards for medical devices utilizing animal tissues and their
derivatives (YY/T 0771.1, YY/T 0771.2, YY/T 0771.3).
5.21 Biological evaluation
The sodium hyaluronate shall be subjected to biological evaluation according to the
requirements of GB/T 16886.1; sodium hyaluronate shall not release substances that
have adverse effects on the human body.
6 Test methods
6.1 Appearance
Perform direct observation with the naked eye, which shall comply with the provisions
of 5.1.
6.2 Fourier transform infrared spectrum (FT-IR)
Prepare the sample by the potassium bromide tablet method, and determine according
to the method specified in the general rule 0402 of the Pharmacopoeia of the People’s
Republic of China (2015 Edition, Volume IV), which shall meet the requirements of 5.2.
6.3 Determination of sodium hyaluronate content
Measure according to the method specified in Appendix A, which shall comply with
the provisions of 5.3.
6.4 pH determination
According to the method stipulated in the general rule 0831 of Pharmacopoeia of the
People’s Republic of China (2015 Edition, Volume IV), take 0.5 g of this product, use
phosphorus pentoxide as a desiccant, and dry it at 105 °C for 6 h for determination,
which shall meet the requirements of 5.10.
6.11 Determination of quaternary ammonium residues
Measure by referring to the method specified in Appendix D, which shall comply with
the provisions of 5.11.
6.12 Determination of sulfated mucopolysaccharides
Test solution: Take about 50.0 mg of this product (based on the dry product); add 1.0
mL of perchloric acid to a stoppered test tube (length is 5 cm; inner diameter is 1.6 cm);
dissolve it; use it as the test solution.
Control solution: Weigh 0.149 g of anhydrous sodium sulfate; add water to dilute to
100 mL, to dissolve it. Then, take 10.0 mL of the above solution; add water to dilute to
100 mL, to obtain it; take 1.0 mL of the solution; put it in a stoppered test tube; heat
and evaporate to dryness at 90 °C ~ 95 °C; then, add 1.0 mL of perchloric acid as a
control solution.
For both the test solution and the control solution, use glass wool to plug the test tube;
heat at 180 °C until clear (about 12 h); then, cool to room temperature. Add 3.0 mL of
33.3 g/L barium chloride solution respectively; shake after sealing; place for 30 min.
After shaking, follow the method specified in the general rule 0401 of Pharmacopoeia
of the People’s Republic of China (2015 Edition, Volume IV); use water as a blank;
measure the absorbance of the test so...
Get QUOTATION in 1-minute: Click YY/T 1571-2017
Historical versions: YY/T 1571-2017
Preview True-PDF (Reload/Scroll if blank)
YY/T 1571-2017: Tissue engineering medical device products—Sodium hyaluronate
YY/T 1571-2017
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.40
C 45
Replacing YY/T 0606.9-2007
Tissue engineering medical device products - Sodium
hyaluronate
组织工程医疗器械产品 透明质酸钠
ISSUED ON: MAY 02, 2017
IMPLEMENTED ON: APRIL 01, 2018
Issued by: China Food and Drug Administration
Table of Contents
Foreword ... 3
1 Scope ... 6
2 Normative references ... 6
3 Terms and definitions ... 7
4 Classification ... 7
5 Requirements ... 8
6 Test methods ... 10
7 Marking ... 14
8 Packaging, transportation and storage ... 15
Appendix A (Normative) Determination of sodium hyaluronate content ... 16
Appendix B (Normative) Determination of protein content ... 19
Appendix C (Normative) Determination of residual ethanol (headspace gas
chromatography) ... 21
Appendix D (Informative) Determination of residual quaternary ammonium salt
(cetylpyridinium chloride) ... 23
Appendix E (Normative) Determination of weight-average molecular weight and
molecular weight distribution coefficient ... 26
References ... 28
Tissue engineering medical device products - Sodium
hyaluronate
1 Scope
This Standard specifies the requirements and test methods for sodium hyaluronate used
in surgical implants and tissue engineering medical device products.
This Standard applies to the preparation of sodium hyaluronate for tissue engineering
medical device products and their scaffold materials.
2 Normative references
The following referenced documents are indispensable for the application of this
document. For dated references, only the edition cited applies to this document. For
undated references, the latest edition (including any amendment) applies to this
document.
GB/T 191, Packaging - Pictorial marking for handling of goods
GB/T 14518, Determination of the pH of adhesives
GB/T 16886.1, Biological evaluation of medical devices - Part 1: Evaluation and
testing within a risk management process (GB/T 16886.1-2011, ISO 10993-1:2009,
IDT)
GB 18278 (all parts), Sterilization of health care products - Moist heat
GB 18279 (all parts), Sterilization of health care products - Ethylene oxide
GB 18280 (all parts), Sterilization of health care products - Radiation
YY/T 0313, Medical polymer products - Requirement for package and information
supplied by manufacturer
YY/T 0606.25, Tissue engineered medical product - Part 25: Quantification of
remnant DNA in biological materials utilizing animal tissues and their derivatives:
Fluorescence method
YY/T 0771.1, Medical devices utilizing animal tissues and their derivatives - Part 1:
Application of risk management (YY/T 0771.1-2009, ISO 22442-1:2007, IDT)
5 Requirements
5.1 Appearance
White or off-white powder or granular or fibrous solid, without any visible foreign
matter.
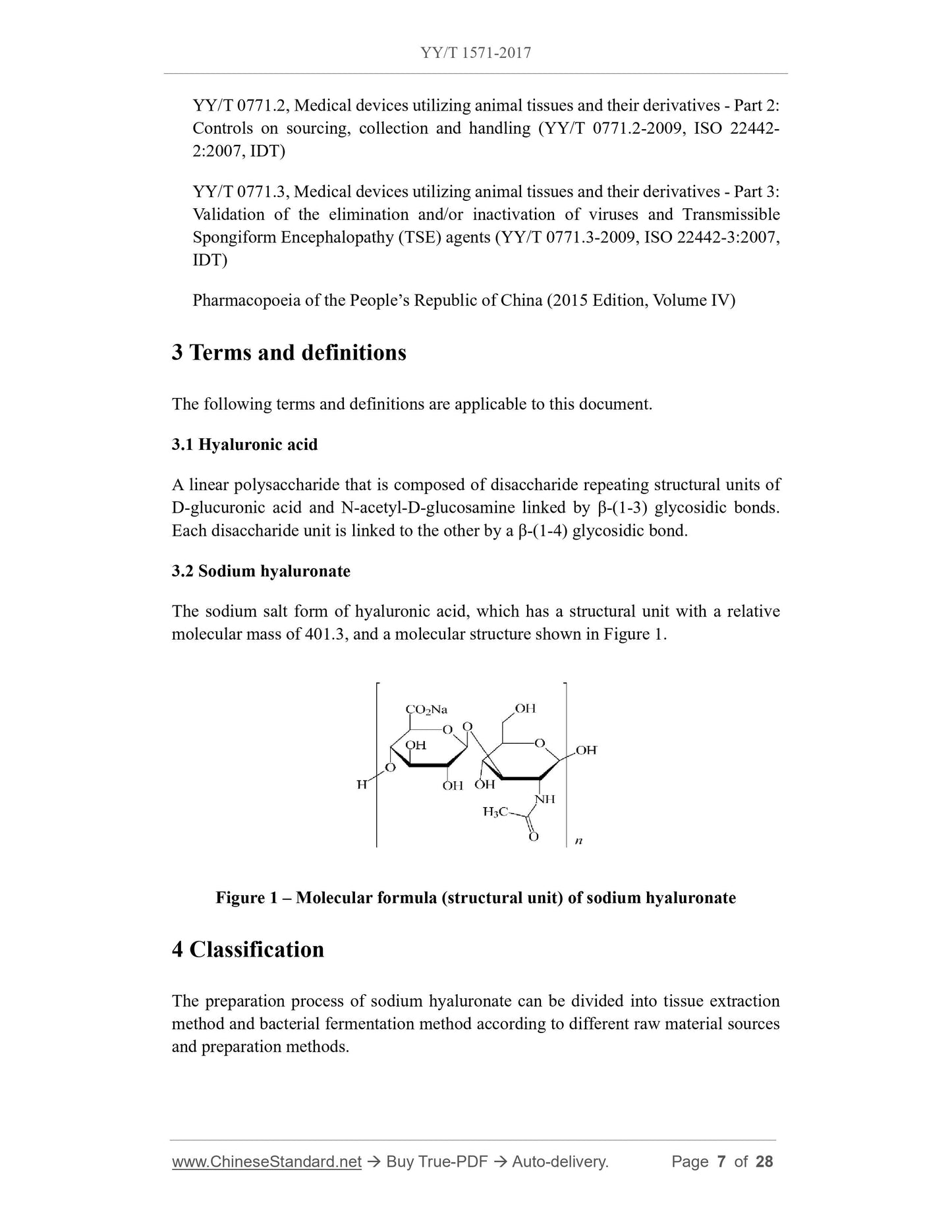
5.2 Identification
Typical Fourier transform infrared spectrum (FT-IR) frequencies (cm-1) of sodium
hyaluronate are 3 275 ~ 3 390(b), 1 615(s), 1 405(m), 1 377(m), 1 150, 1 077, 1 045(s),
946(m), 893(w). The wavenumber error of the measured band in the fingerprint area
shall be less than the specified value ±5 cm-1 (0.5%).
Note: s, strong; m, medium; w, weak; b, broad.
5.3 Sodium hyaluronate content
Based on the dry product, the content of sodium hyaluronate shall be 95.0% ~ 105.0%
(mass fraction).
5.4 pH
The pH of the 0.5% concentration solution shall be 5.0 ~ 8.5.
5.5 Intrinsic viscosity
It shall be 90% ~ 120% of its marked value.
5.6 Protein content
It shall not exceed 0.1% (mass fraction).
5.7 Nucleic acid content
5.7.1 Bacterial fermentation method
For solution of the concentration 0.33%, the absorbance value (OD value) at the
wavelength of 260 nm shall not exceed 0.5.
5.7.2 Tissue extraction method
In accordance with YY/T 0606.25, the limit value of residual DNA is given according
to the possible intended use.
5.8 Heavy metal content
It shall not exceed 10 μg/g (mass fraction).
The number of bacterial colonies per 1 g of the test product shall not exceed 102 CFU;
the number of mold and yeast colonies shall not exceed 20 CFU. Staphylococcus aureus,
Pseudomonas aeruginosa and Escherichia coli shall not be detected.
Note: If sodium hyaluronate is provided in a non-sterile manner, this item shall be tested;
if the final product is required to be sterile, it should be sterilized to meet the
sterile requirements.
5.19 Bacterial endotoxins
The amount of endotoxin contained per 1 mg of sodium hyaluronate shall be less than
0.05 EU.
5.20 Raw material safety
5.20.1 The sodium hyaluronate prepared by the bacterial fermentation method shall be
tested for hemolytic streptolysin, and the result shall be free from hemolytic ring.
5.20.2 The sodium hyaluronate prepared by the tissue extraction method shall be
subjected to safety evaluation and quality control in accordance with the requirements
of the series industry standards for medical devices utilizing animal tissues and their
derivatives (YY/T 0771.1, YY/T 0771.2, YY/T 0771.3).
5.21 Biological evaluation
The sodium hyaluronate shall be subjected to biological evaluation according to the
requirements of GB/T 16886.1; sodium hyaluronate shall not release substances that
have adverse effects on the human body.
6 Test methods
6.1 Appearance
Perform direct observation with the naked eye, which shall comply with the provisions
of 5.1.
6.2 Fourier transform infrared spectrum (FT-IR)
Prepare the sample by the potassium bromide tablet method, and determine according
to the method specified in the general rule 0402 of the Pharmacopoeia of the People’s
Republic of China (2015 Edition, Volume IV), which shall meet the requirements of 5.2.
6.3 Determination of sodium hyaluronate content
Measure according to the method specified in Appendix A, which shall comply with
the provisions of 5.3.
6.4 pH determination
According to the method stipulated in the general rule 0831 of Pharmacopoeia of the
People’s Republic of China (2015 Edition, Volume IV), take 0.5 g of this product, use
phosphorus pentoxide as a desiccant, and dry it at 105 °C for 6 h for determination,
which shall meet the requirements of 5.10.
6.11 Determination of quaternary ammonium residues
Measure by referring to the method specified in Appendix D, which shall comply with
the provisions of 5.11.
6.12 Determination of sulfated mucopolysaccharides
Test solution: Take about 50.0 mg of this product (based on the dry product); add 1.0
mL of perchloric acid to a stoppered test tube (length is 5 cm; inner diameter is 1.6 cm);
dissolve it; use it as the test solution.
Control solution: Weigh 0.149 g of anhydrous sodium sulfate; add water to dilute to
100 mL, to dissolve it. Then, take 10.0 mL of the above solution; add water to dilute to
100 mL, to obtain it; take 1.0 mL of the solution; put it in a stoppered test tube; heat
and evaporate to dryness at 90 °C ~ 95 °C; then, add 1.0 mL of perchloric acid as a
control solution.
For both the test solution and the control solution, use glass wool to plug the test tube;
heat at 180 °C until clear (about 12 h); then, cool to room temperature. Add 3.0 mL of
33.3 g/L barium chloride solution respectively; shake after sealing; place for 30 min.
After shaking, follow the method specified in the general rule 0401 of Pharmacopoeia
of the People’s Republic of China (2015 Edition, Volume IV); use water as a blank;
measure the absorbance of the test so...
Share