1
/
の
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1797-2021 English PDF (YYT1797-2021)
YY/T 1797-2021 English PDF (YYT1797-2021)
通常価格
$320.00 USD
通常価格
セール価格
$320.00 USD
単価
/
あたり
配送料はチェックアウト時に計算されます。
受取状況を読み込めませんでした
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY/T 1797-2021
Historical versions: YY/T 1797-2021
Preview True-PDF (Reload/Scroll if blank)
YY/T 1797-2021: Endoscopic surgical instruments - Endoscopic cutter stapler and reload
YY/T 1797-2021
YY
PHARMACEUTICAL INDUSTRY STANDARD
ICS 11.040.30
C 36
Endoscopic surgical instruments - Endoscopic cutter stapler
and reload
ISSUED ON. SEPTEMBER 06, 2021
IMPLEMENTED ON. SEPTEMBER 01, 2022
Issued by. National Medical Products Administration
Table of Contents
Foreword... 3
1 Scope... 4
2 Normative references... 4
3 Structure and materials... 5
4 Requirements... 9
5 Test methods... 13
6 Type inspection... 17
7 Label, instruction manual... 17
8 Packaging... 19
Appendix A (Informative) Test materials for anastomosis and cutting performance.. 20
Appendix B (Normative) Staple line suture strength test... 21
Appendix C (Normative) Test method for cutting edge sharpness... 23
Appendix D (Normative) Pressure performance test... 26
Endoscopic surgical instruments - Endoscopic cutter stapler
and reload
1 Scope
This Standard specifies the structure and materials, requirements, test methods, type
inspection, labels, instruction manuals and packaging of endoscopic cutter stapler and
reload (hereinafter referred to as stapler) used in endoscopic surgery.
This Standard applies to disposable endoscopic cutter stapler and reload used in
endoscopic surgery.
Note. This stapler applies to anastomotic establishment and stump or incision closure
in digestive tract reconstruction and organ resection.
2 Normative references
The following referenced documents are indispensable for the application of this
document. For dated references, only the edition cited applies to this document. For
undated references, the latest edition (including any amendment) applies to this
document.
GB/T 228.1, Metallic materials - Tensile testing - Part 1.Method of test at room
temperature
GB/T 1220, Stainless steel bars
GB/T 3280, Cold rolled stainless steel plate, sheet and strip
GB/T 4237, Hot rolled stainless steel plate, sheet and strip
GB/T 4340.1, Metallic materials - Vickers hardness test. Part 1.Test method
GB/T 6682-2008, Water for analytical laboratory use - Specification and test
methods
GB/T 10610, Geometrical product specifications (GPS) - Surface texture. Profile
method - Rules and procedures for the assessment of surface texture
GB/T 13810, Wrought titanium and titanium alloy for surgical implants
GB/T 14233.1-2008, Test methods for infusion, transfusion, injection equipment for
medical use - Part 1.Chemical analysis methods
GB/T 16886, Biological evaluation of medical devices
YY/T 0149-2006, Medical instruments of stainless steel - Test methods of corrosion
resistance
YY 0167-2005, Non-absorbable surgical suture
Pharmacopoeia of the People’s Republic of China
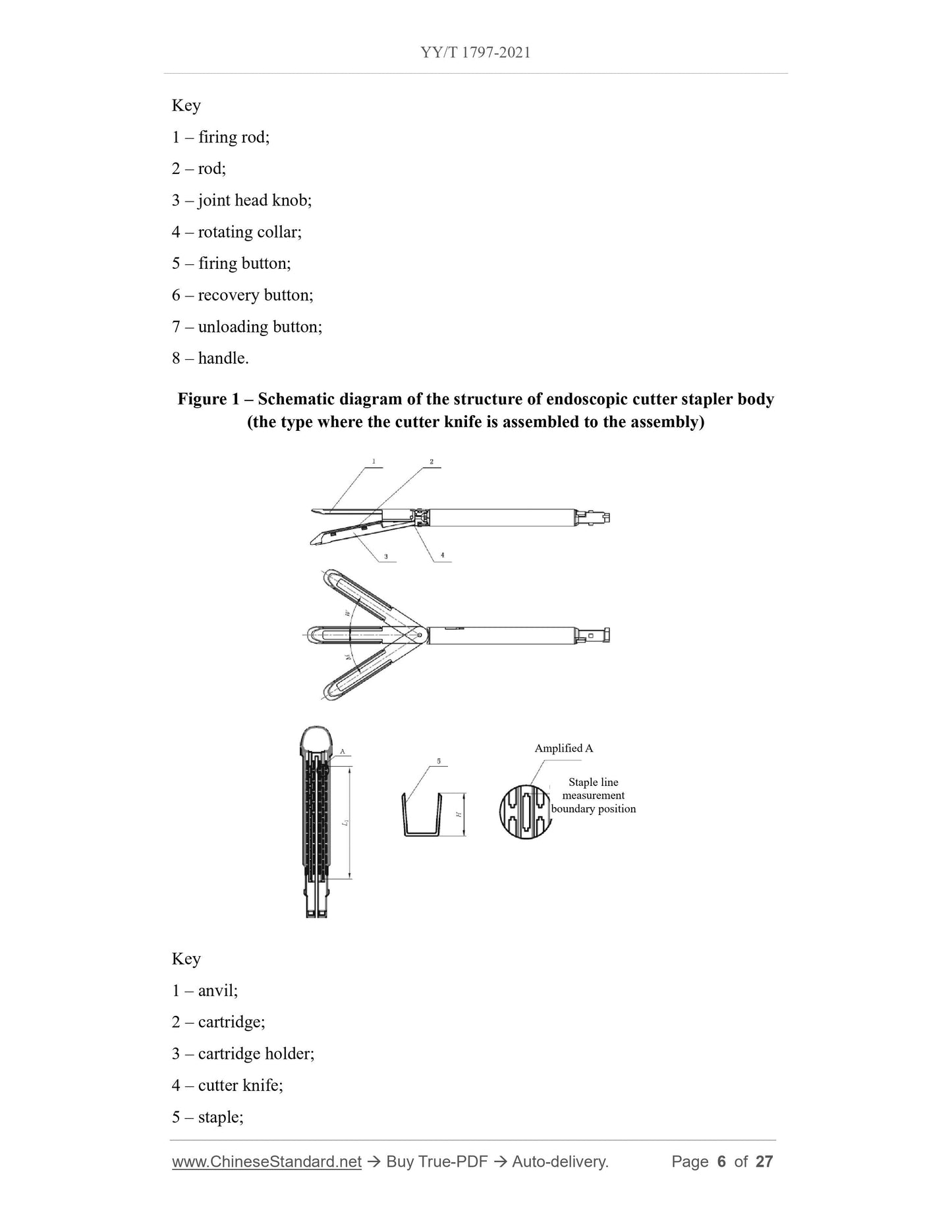
3 Structure and materials
3.1 The stapler consists of a stapler body and some assemblies. According to the design
of the cutter knife, the stapler can be divided into a type where the cutter knife is
assembled to the assembly and a type where the cutter knife is assembled to the stapler
body; according to the joint structure, it can be divided into the bending type and the
non-bending type.
For the endoscopic cutter stapler whose cutter knife is assembled to the assembly, the
stapler body is generally composed of a firing rod, a rod, a joint head knob, a rotating
collar, a firing button, a recovery button, an unloading button, a handle, etc.; the
assemblies consist of an anvil, a cartridge, a cartridge holder, a cutter and staples.
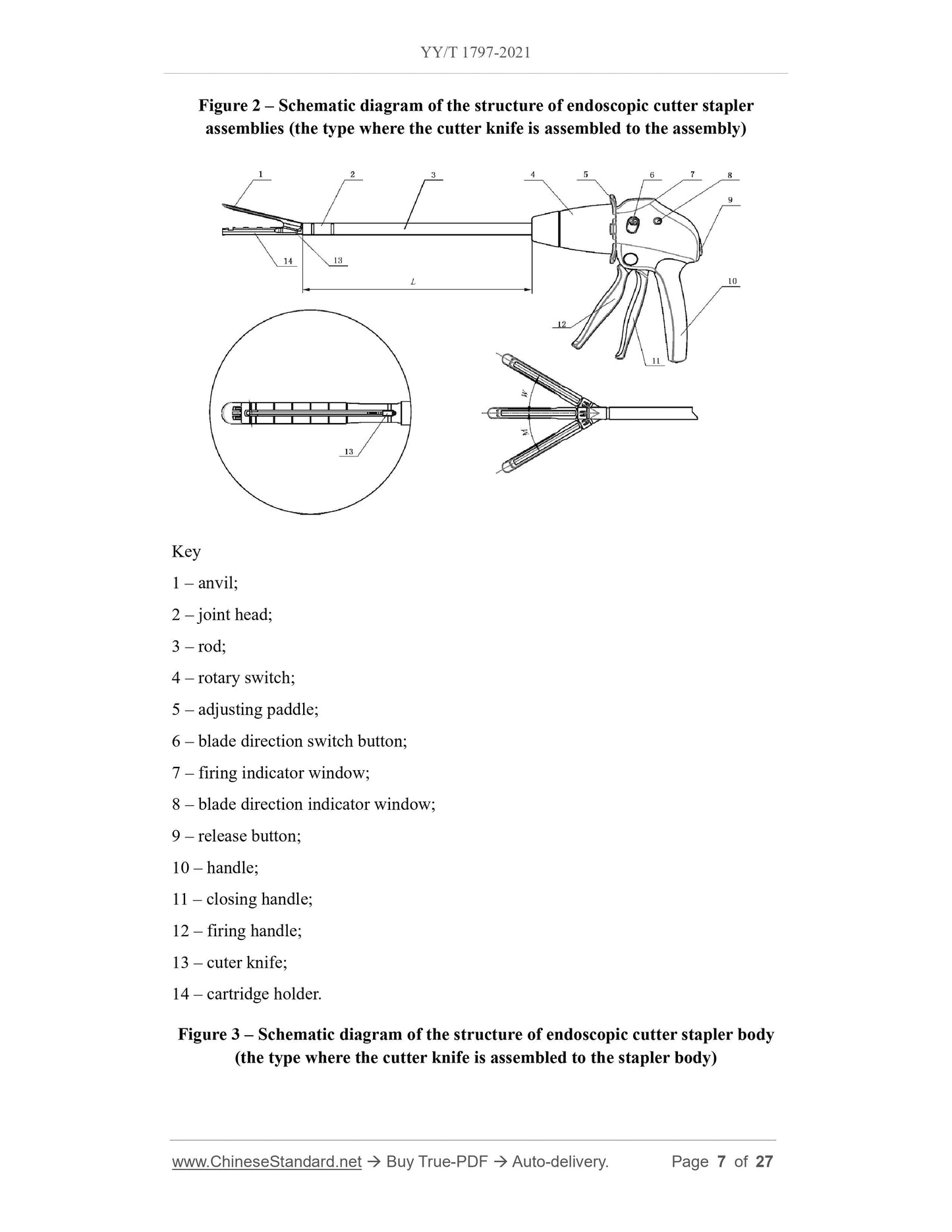
For the endoscopic cutter stapler whose cutter knife is assembled to the stapler body,
the stapler body is generally composed of an anvil, a joint head, a rod, a rotary knob,
an adjusting paddle, a blade direction switch button, a firing indicator window, a blade
direction indicator window, a release button, a handle, a closing handle, a firing handle,
a cutter knife, a cartridge holder, etc.; the assemblies are generally consist of a cartridge
and staples.
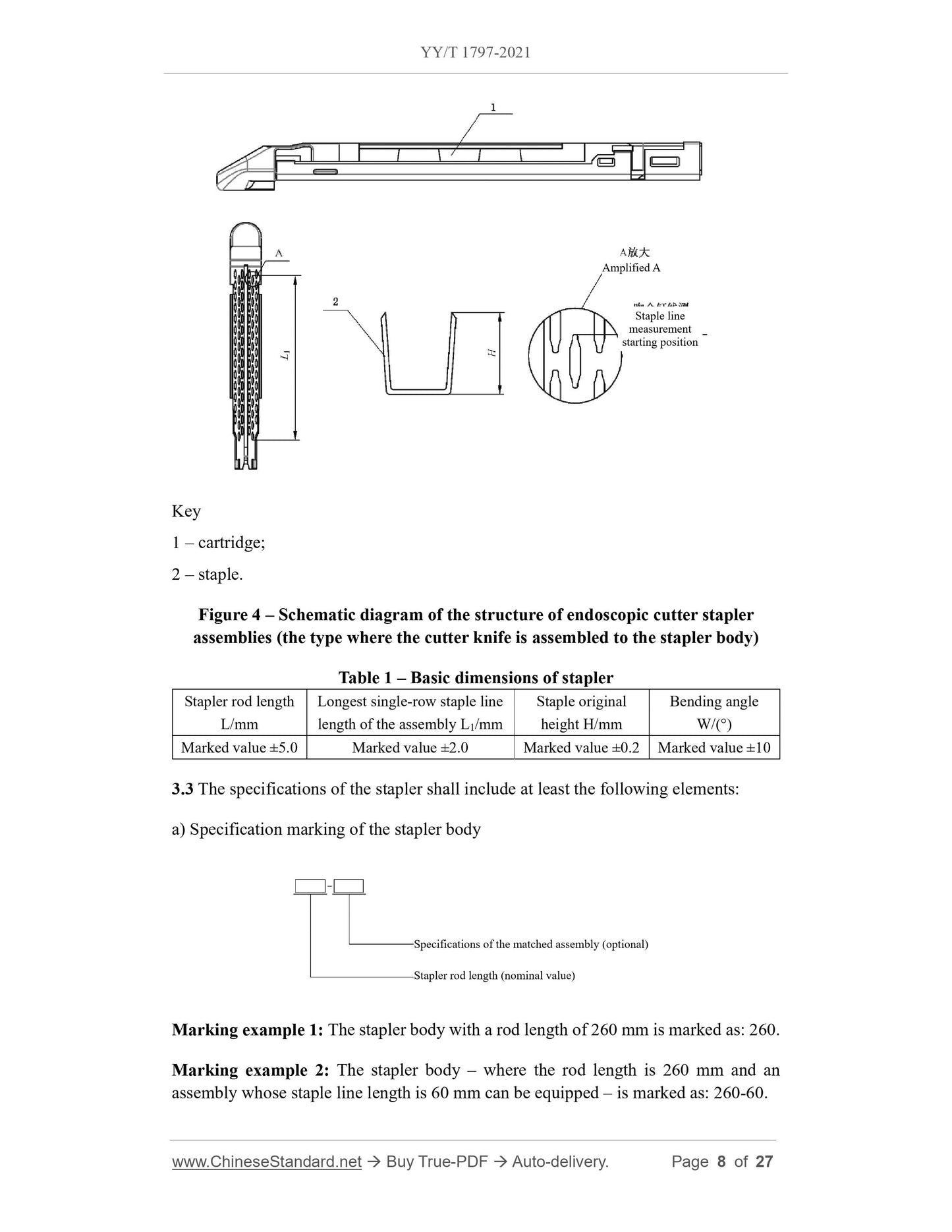
3.2 See Figure 1, Figure 2, Figure 3, Figure 4 and Table 1 for the structure and basic
dimensions of the stapler.
Note. The structure of the stapler shown is not the only type.
4.2.2 The shape and structure of the stapler head end shall be blunt, without sharp edges
or corners.
4.2.3 The surface of the stapler (assemblies and stapler rod parts) shall be matt.
4.2.4 The handwriting and marking on the outer surface of the stapler shall be clear and
free of defects such as dislocation and skew.
4.2.5 The tip of the staple shall be sharp, and the surface shall be free of defects such
as burrs and dents.
4.3 Corrosion resistance
The corrosion resistance of the metal parts on the outer surface of the stapler head end
shall not be lower than that specified in 5.4b) of YY/T 0149-2006.
4.4 Surface roughness
The roughness Ra of the metal outer surface of the stapler assemblies and the stapler
rod parts shall not be greater than 1.6 μm.
4.5 Dimensions
The basic dimensions of the stapler shall meet the requirements of Table 1.
4.6 Hardness
The hardness of the cutter knife shall not be less than 377HV0.2.
4.7 Assembly
4.7.1 The stapler shall be positioned accurately; the replacement of assemblies shall be
convenient, firm, and non-blocking; there shall be a sound or other prompts when the
assemblies are installed in place.
4.7.2 The staples shall be stably loaded into the assembly, and the staples shall not be
exposed on the surface of the staple cartridge after being shaken.
4.8 Flexibility
4.8.1 The opening and closing of the stapler jaws shall be flexible, and there shall be
no blocking phenomenon.
4.8.2 The joint structure and rotational structure of the stapler shall be flexible and free
of obstacles.
4.9 Performance
4.9.1 The connection between the stapler body and the assemblies shall be firm and
reliable.
4.9.2 The bending stapler, after swinging to the maximum angle, shall be able to
successfully complete the firing and reset.
4.9.3 The jaws of the stapler, after being closed, shall have a certain clamping force,
which shall comply with the regulations of the enterprise.
4.9.4 The jaws of the stapler, after being closed, shall have a certain closing force, which
shall comply with the regulations of the enterprise.
4.9.5 The stapler shall be available for one-hand operability.
4.9.6 The stapler shall be provided with good stapling and cutting performance. When
the assemblies are replaced, the cutting edge after each anastomosis shall be neat and
free of burrs, and the distal-end length of the staple line shall be at least 1.5 times longer
than the cutting line. The staples after each anastomosis shall be shaped like "B".
4.9.7 The staple line after anastomosis shall have certain staple line suture strength, and
the staple line suture strength shall comply with the regulations of the enterprise.
4.9.8 The stapler shall be provided with a firing process feedback indicating device that
can indicate the firing process or state.
4.10 Cutter knife sharpness
The cutting edge of the cutter knife shall be sharp, and there shall be no curling or
chipping; the cutting force shall not be greater than 0.80 N.
4.11 Pressure resistance
The stapled anastomosis or suture shall be able to withstand a pressure of not less than
3.6×103 Pa, and the leakage of water shall not exceed 10 drops within 15 s.
4.12 Protective device
4.12.1 The stapler shall have an empty staple cartridge protective device, so that it
cannot be fired when the empty staple cartridge is installed by mistake.
Note. Empty car...
Get QUOTATION in 1-minute: Click YY/T 1797-2021
Historical versions: YY/T 1797-2021
Preview True-PDF (Reload/Scroll if blank)
YY/T 1797-2021: Endoscopic surgical instruments - Endoscopic cutter stapler and reload
YY/T 1797-2021
YY
PHARMACEUTICAL INDUSTRY STANDARD
ICS 11.040.30
C 36
Endoscopic surgical instruments - Endoscopic cutter stapler
and reload
ISSUED ON. SEPTEMBER 06, 2021
IMPLEMENTED ON. SEPTEMBER 01, 2022
Issued by. National Medical Products Administration
Table of Contents
Foreword... 3
1 Scope... 4
2 Normative references... 4
3 Structure and materials... 5
4 Requirements... 9
5 Test methods... 13
6 Type inspection... 17
7 Label, instruction manual... 17
8 Packaging... 19
Appendix A (Informative) Test materials for anastomosis and cutting performance.. 20
Appendix B (Normative) Staple line suture strength test... 21
Appendix C (Normative) Test method for cutting edge sharpness... 23
Appendix D (Normative) Pressure performance test... 26
Endoscopic surgical instruments - Endoscopic cutter stapler
and reload
1 Scope
This Standard specifies the structure and materials, requirements, test methods, type
inspection, labels, instruction manuals and packaging of endoscopic cutter stapler and
reload (hereinafter referred to as stapler) used in endoscopic surgery.
This Standard applies to disposable endoscopic cutter stapler and reload used in
endoscopic surgery.
Note. This stapler applies to anastomotic establishment and stump or incision closure
in digestive tract reconstruction and organ resection.
2 Normative references
The following referenced documents are indispensable for the application of this
document. For dated references, only the edition cited applies to this document. For
undated references, the latest edition (including any amendment) applies to this
document.
GB/T 228.1, Metallic materials - Tensile testing - Part 1.Method of test at room
temperature
GB/T 1220, Stainless steel bars
GB/T 3280, Cold rolled stainless steel plate, sheet and strip
GB/T 4237, Hot rolled stainless steel plate, sheet and strip
GB/T 4340.1, Metallic materials - Vickers hardness test. Part 1.Test method
GB/T 6682-2008, Water for analytical laboratory use - Specification and test
methods
GB/T 10610, Geometrical product specifications (GPS) - Surface texture. Profile
method - Rules and procedures for the assessment of surface texture
GB/T 13810, Wrought titanium and titanium alloy for surgical implants
GB/T 14233.1-2008, Test methods for infusion, transfusion, injection equipment for
medical use - Part 1.Chemical analysis methods
GB/T 16886, Biological evaluation of medical devices
YY/T 0149-2006, Medical instruments of stainless steel - Test methods of corrosion
resistance
YY 0167-2005, Non-absorbable surgical suture
Pharmacopoeia of the People’s Republic of China
3 Structure and materials
3.1 The stapler consists of a stapler body and some assemblies. According to the design
of the cutter knife, the stapler can be divided into a type where the cutter knife is
assembled to the assembly and a type where the cutter knife is assembled to the stapler
body; according to the joint structure, it can be divided into the bending type and the
non-bending type.
For the endoscopic cutter stapler whose cutter knife is assembled to the assembly, the
stapler body is generally composed of a firing rod, a rod, a joint head knob, a rotating
collar, a firing button, a recovery button, an unloading button, a handle, etc.; the
assemblies consist of an anvil, a cartridge, a cartridge holder, a cutter and staples.
For the endoscopic cutter stapler whose cutter knife is assembled to the stapler body,
the stapler body is generally composed of an anvil, a joint head, a rod, a rotary knob,
an adjusting paddle, a blade direction switch button, a firing indicator window, a blade
direction indicator window, a release button, a handle, a closing handle, a firing handle,
a cutter knife, a cartridge holder, etc.; the assemblies are generally consist of a cartridge
and staples.
3.2 See Figure 1, Figure 2, Figure 3, Figure 4 and Table 1 for the structure and basic
dimensions of the stapler.
Note. The structure of the stapler shown is not the only type.
4.2.2 The shape and structure of the stapler head end shall be blunt, without sharp edges
or corners.
4.2.3 The surface of the stapler (assemblies and stapler rod parts) shall be matt.
4.2.4 The handwriting and marking on the outer surface of the stapler shall be clear and
free of defects such as dislocation and skew.
4.2.5 The tip of the staple shall be sharp, and the surface shall be free of defects such
as burrs and dents.
4.3 Corrosion resistance
The corrosion resistance of the metal parts on the outer surface of the stapler head end
shall not be lower than that specified in 5.4b) of YY/T 0149-2006.
4.4 Surface roughness
The roughness Ra of the metal outer surface of the stapler assemblies and the stapler
rod parts shall not be greater than 1.6 μm.
4.5 Dimensions
The basic dimensions of the stapler shall meet the requirements of Table 1.
4.6 Hardness
The hardness of the cutter knife shall not be less than 377HV0.2.
4.7 Assembly
4.7.1 The stapler shall be positioned accurately; the replacement of assemblies shall be
convenient, firm, and non-blocking; there shall be a sound or other prompts when the
assemblies are installed in place.
4.7.2 The staples shall be stably loaded into the assembly, and the staples shall not be
exposed on the surface of the staple cartridge after being shaken.
4.8 Flexibility
4.8.1 The opening and closing of the stapler jaws shall be flexible, and there shall be
no blocking phenomenon.
4.8.2 The joint structure and rotational structure of the stapler shall be flexible and free
of obstacles.
4.9 Performance
4.9.1 The connection between the stapler body and the assemblies shall be firm and
reliable.
4.9.2 The bending stapler, after swinging to the maximum angle, shall be able to
successfully complete the firing and reset.
4.9.3 The jaws of the stapler, after being closed, shall have a certain clamping force,
which shall comply with the regulations of the enterprise.
4.9.4 The jaws of the stapler, after being closed, shall have a certain closing force, which
shall comply with the regulations of the enterprise.
4.9.5 The stapler shall be available for one-hand operability.
4.9.6 The stapler shall be provided with good stapling and cutting performance. When
the assemblies are replaced, the cutting edge after each anastomosis shall be neat and
free of burrs, and the distal-end length of the staple line shall be at least 1.5 times longer
than the cutting line. The staples after each anastomosis shall be shaped like "B".
4.9.7 The staple line after anastomosis shall have certain staple line suture strength, and
the staple line suture strength shall comply with the regulations of the enterprise.
4.9.8 The stapler shall be provided with a firing process feedback indicating device that
can indicate the firing process or state.
4.10 Cutter knife sharpness
The cutting edge of the cutter knife shall be sharp, and there shall be no curling or
chipping; the cutting force shall not be greater than 0.80 N.
4.11 Pressure resistance
The stapled anastomosis or suture shall be able to withstand a pressure of not less than
3.6×103 Pa, and the leakage of water shall not exceed 10 drops within 15 s.
4.12 Protective device
4.12.1 The stapler shall have an empty staple cartridge protective device, so that it
cannot be fired when the empty staple cartridge is installed by mistake.
Note. Empty car...
Share