1
/
의
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY 0789-2010 English PDF (YY0789-2010)
YY 0789-2010 English PDF (YY0789-2010)
정가
$155.00 USD
정가
할인가
$155.00 USD
단가
/
단위
배송료는 결제 시 계산됩니다.
픽업 사용 가능 여부를 로드할 수 없습니다.
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY 0789-2010
Historical versions: YY 0789-2010
Preview True-PDF (Reload/Scroll if blank)
YY 0789-2010: [Including 2021XG1] Q-Switched Nd: YAG laser ophthalmic system
YY 0789-2010
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.70
C 41
Q-Switched Nd: YAG Laser Ophthalmic System
ISSUED ON: DECEMBER 27, 2010
IMPLEMENTED ON: JUNE 1, 2012
Issued by: National Medical Products Administration
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative References ... 4
3 Product Composition and Basic Parameters ... 4
4 Requirements ... 5
5 Test Methods ... 8
6 Inspection Rules ... 11
7 Marking, Labeling and Instruction Manual ... 12
8 Packaging, Transportation and Storage ... 14
YY 0789-2010 Q-Switched Nd: YAG Laser Ophthalmic System No.1 Amendment
[2021XG1] ... 15
Q-Switched Nd: YAG Laser Ophthalmic System
1 Scope
This Standard specifies the basic parameters, product composition, technical requirements, test
methods, as well as marking and labeling, and packaging requirements for Q-switched Nd:
YAG laser ophthalmic system.
This Standard is applicable to Q-switched Nd: YAG laser ophthalmic system (hereinafter
referred to as ophthalmic system). The ophthalmic system is used for the incision and resection
of the anterior segment tissue through the photolysis / photo-blasting effect of the laser pulse
with a wavelength of 1,064 nm to achieve the purpose of treatment.
2 Normative References
The clauses of the following documents become clauses of this Standard through the normative
references in this Standard. In terms of references with a specified date, all subsequent
amendments (excluding errata content) or revisions do not apply to this Standard. However, the
various parties that reach an agreement in accordance with this Standard are encouraged to
explore whether the latest versions of these documents are applicable. In terms of references
without a date, the latest versions apply to this Standard.
GB/T 191-2008 Packaging - Pictorial Marking for Handling of Goods (ISO 780:1997, MOD)
GB 7247.1-2001 Safety of Laser Products - Part 1: Equipment Classification, Requirements
and User’s Guide (idt IEC 60825-1:1993)
GB 9706.1-2007 Medical Electrical Equipment - Part 1: General Requirements for Safety (IEC
60601-1:1988, IDT)
GB 9706.20-2000 Medical Electrical Equipment - Part 2: Particular Requirements for the
Safety of Diagnostic and Therapeutic Laser Equipment (idt IEC 60601-2-22:1995)
GB/T 14710-2009 Environmental Requirement and Test Methods for Medical Electrical
Equipment
YY 0065-2007 Ophthalmic Instruments - Slit-lamp Microscopes
3 Product Composition and Basic Parameters
3.1 The constituent parts of the ophthalmic system:
a) Q-switched Nd: YAG laser;
b) Power supply and control system;
c) Safety and protection system;
d) Slit-lamp microscope adapter.
3.2 Basic parameters of the ophthalmic system:
The manufacturer shall list the following basic parameters in the registered product standards:
a) Therapeutic laser wavelength;
b) Maximum output energy of therapeutic laser pulse / pulse train;
c) Duration of therapeutic laser pulse;
d) Focal spot diameter of therapeutic laser output;
e) Aiming light wavelength;
f) Aiming light power;
g) Optical parameters of slit-lamp microscope adapter.
4 Requirements
4.1 Normal Operating Conditions
The ophthalmic system shall be able to normally operate under the following conditions:
---Ambient temperature: 10 C ~ 30 C;
---Relative humidity: not greater than 70%;
---Atmospheric pressure: 86.0 kPa ~ 106.0 kPa;
---Power supply used: AC 220 V/(100 V ~ 240 V), 50 Hz/60 Hz;
---There is no strong electromagnetic field interference around:
---There is no obvious vibration or disturbing airflow around.
4.2 Therapeutic Laser
4.2.1 Laser wavelength
Laser wavelength is 1,064 nm 5 nm.
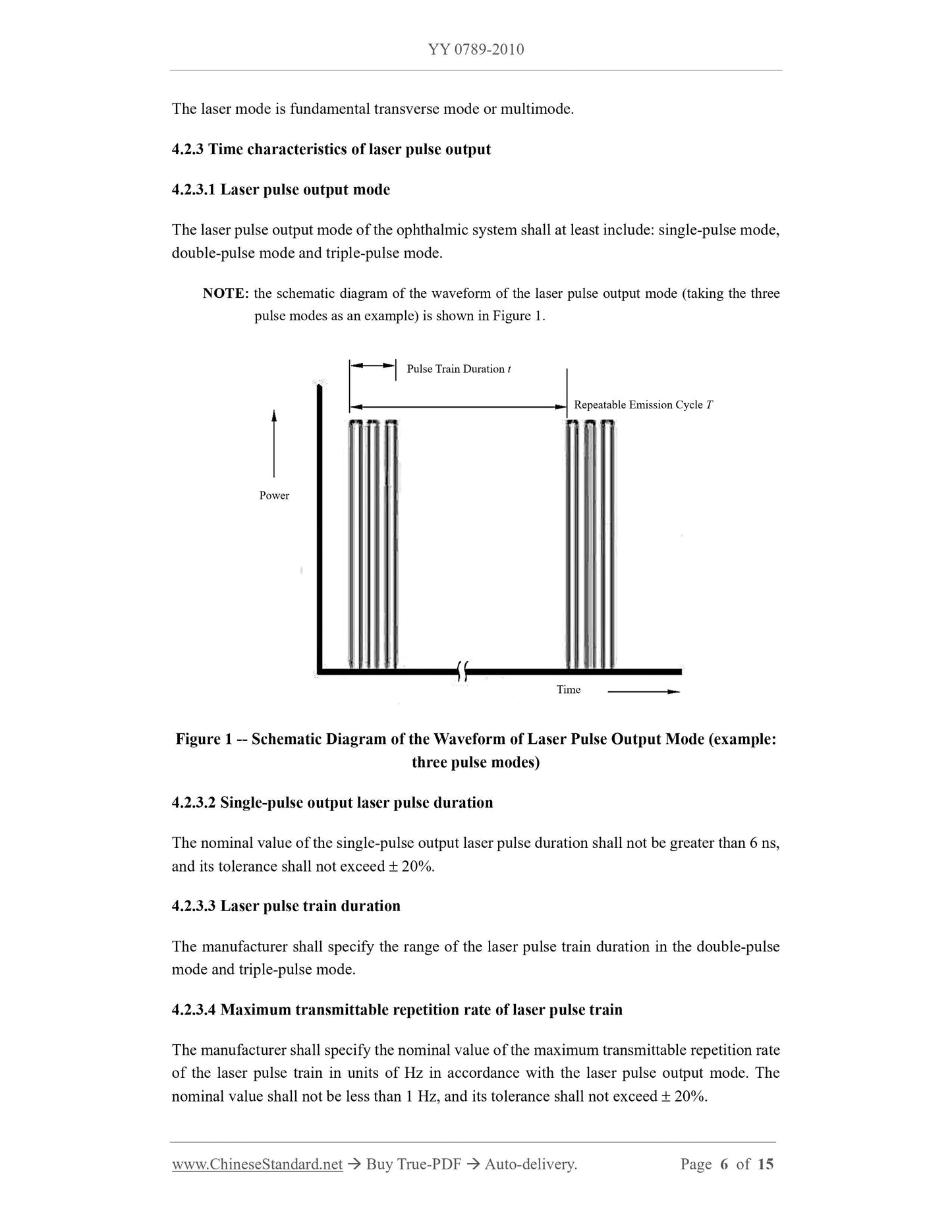
4.2.2 Laser mode
4.2.4 Terminal output energy of laser pulse / pulse train
4.2.4.1 Maximum terminal output energy
The manufacturer shall specify the nominal value of the maximum terminal output energy of
the laser pulse / pulse train in accordance with the laser pulse output mode, and its tolerance
shall not exceed 20%.
4.2.4.2 Terminal output energy regulation
The terminal output energy shall be adjustable. The manufacturer shall provide the range of
adjustment, and its tolerance shall not exceed 20%.
4.2.5 Reproducibility of laser output energy
It shall not exceed 20%.
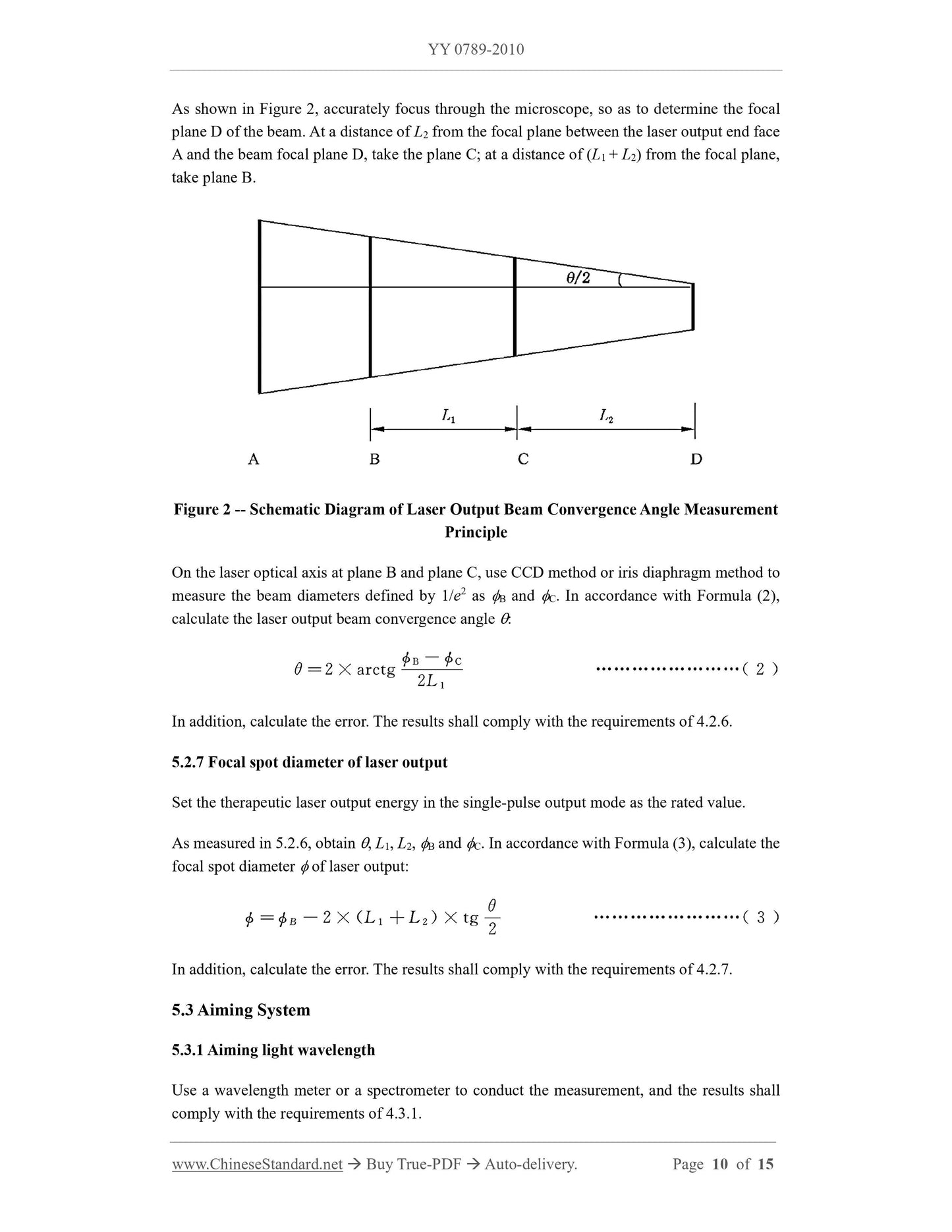
4.2.6 Laser output beam convergence angle
The manufacturer shall specify the nominal value and tolerance (shall not exceed 20%) of the
laser output beam convergence angle, or the range of the beam convergence angle; the beam
convergence angle shall not be less than 14.
4.2.7 Focal spot diameter of laser output
The manufacturer shall specify the nominal value and tolerance (shall not exceed 20%) of the
focal spot diameter of laser output, or the range of focal spot diameter; the focal spot diameter
shall not be greater than 50 m.
4.3 Aiming System
4.3.1 Aiming light wavelength. The manufacturer shall specify the nominal value and tolerance
of the central wavelength of the aiming light, or the range of the central wavelength.
4.3.2 Aiming light power. It shall not be greater than 1 mW.
4.3.3 Coincidence of aiming light. The aiming light and the therapeutic laser shall coincide at
the focal plane.
4.4 Slit-lamp Microscope and Laser Radiation at the Eyepiece
4.4.1 The manufacturer shall specify the performance requirements of the slit-lamp microscope
in accordance with the applicable clauses of YY 0065-2007.
4.4.2 The laser radiation at the eyepiece of the slit-lamp microscope shall not exceed AEL value
of Class I in GB 7247.1-2001.
4.5 Appearance
4.5.1 The appearance shall be clean, uniform in color, and free from corrosion, coating peeling,
scars, scratches, deformation and other defects.
4.5.2 The characters and marks shall be clearly visible.
4.5.3 The control and adjustment mechanism shall be flexible and reliable, the fastening parts
shall not be loose, the button switch shall have a clear hand feeling, and the actions shall be
reliable.
4.6 Safety
The ophthalmic system shall comply with the requirements of GB 9706.1-2007, GB 9706.20-
2000 and GB 7247.1-2001.
The manufacturer shall respectively specify the safety characteristics (including insulation
diagrams) and applicable terms in accordance with GB 9706.1-2007, GB 9706.20-2000 and GB
7247.1-2001.
4.7 Environmental Adaptability
The environmental adaptability of the ophthalmic system shall comply with the requirements
of GB/T 14710-1993; the manufacturer shall provide the specific test conditions and inspection
items. The inspection items shall at least include the maximum terminal output energy in 4.2.4.1.
5 Test Methods
5.1 General
The test methods listed in this Chapter are recommended. If the same effect can be obtained,
other test methods are also allowed to be used.
5.2 Therapeutic Laser
5.2.1 Laser wavelength
Use a wavelength meter or a spectrometer to conduct the measurement, and calculate the error.
The results shall comply with the requirements of 4.2.1.
5.2.2 Laser mode
Use a laser beam analyzer to conduct the inspection. The results shall comply with the
requirements of 4.2.2.
5.2.3 Time characteristics of laser pulse output
The test method for the time characteristics is:
a) Use a photoelectric probe and an oscilloscope to carry out the test, and record the
5.3.2 Laser power of aiming light
Use a laser power meter to conduct the measurement, and the results shall com...
Get QUOTATION in 1-minute: Click YY 0789-2010
Historical versions: YY 0789-2010
Preview True-PDF (Reload/Scroll if blank)
YY 0789-2010: [Including 2021XG1] Q-Switched Nd: YAG laser ophthalmic system
YY 0789-2010
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.70
C 41
Q-Switched Nd: YAG Laser Ophthalmic System
ISSUED ON: DECEMBER 27, 2010
IMPLEMENTED ON: JUNE 1, 2012
Issued by: National Medical Products Administration
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative References ... 4
3 Product Composition and Basic Parameters ... 4
4 Requirements ... 5
5 Test Methods ... 8
6 Inspection Rules ... 11
7 Marking, Labeling and Instruction Manual ... 12
8 Packaging, Transportation and Storage ... 14
YY 0789-2010 Q-Switched Nd: YAG Laser Ophthalmic System No.1 Amendment
[2021XG1] ... 15
Q-Switched Nd: YAG Laser Ophthalmic System
1 Scope
This Standard specifies the basic parameters, product composition, technical requirements, test
methods, as well as marking and labeling, and packaging requirements for Q-switched Nd:
YAG laser ophthalmic system.
This Standard is applicable to Q-switched Nd: YAG laser ophthalmic system (hereinafter
referred to as ophthalmic system). The ophthalmic system is used for the incision and resection
of the anterior segment tissue through the photolysis / photo-blasting effect of the laser pulse
with a wavelength of 1,064 nm to achieve the purpose of treatment.
2 Normative References
The clauses of the following documents become clauses of this Standard through the normative
references in this Standard. In terms of references with a specified date, all subsequent
amendments (excluding errata content) or revisions do not apply to this Standard. However, the
various parties that reach an agreement in accordance with this Standard are encouraged to
explore whether the latest versions of these documents are applicable. In terms of references
without a date, the latest versions apply to this Standard.
GB/T 191-2008 Packaging - Pictorial Marking for Handling of Goods (ISO 780:1997, MOD)
GB 7247.1-2001 Safety of Laser Products - Part 1: Equipment Classification, Requirements
and User’s Guide (idt IEC 60825-1:1993)
GB 9706.1-2007 Medical Electrical Equipment - Part 1: General Requirements for Safety (IEC
60601-1:1988, IDT)
GB 9706.20-2000 Medical Electrical Equipment - Part 2: Particular Requirements for the
Safety of Diagnostic and Therapeutic Laser Equipment (idt IEC 60601-2-22:1995)
GB/T 14710-2009 Environmental Requirement and Test Methods for Medical Electrical
Equipment
YY 0065-2007 Ophthalmic Instruments - Slit-lamp Microscopes
3 Product Composition and Basic Parameters
3.1 The constituent parts of the ophthalmic system:
a) Q-switched Nd: YAG laser;
b) Power supply and control system;
c) Safety and protection system;
d) Slit-lamp microscope adapter.
3.2 Basic parameters of the ophthalmic system:
The manufacturer shall list the following basic parameters in the registered product standards:
a) Therapeutic laser wavelength;
b) Maximum output energy of therapeutic laser pulse / pulse train;
c) Duration of therapeutic laser pulse;
d) Focal spot diameter of therapeutic laser output;
e) Aiming light wavelength;
f) Aiming light power;
g) Optical parameters of slit-lamp microscope adapter.
4 Requirements
4.1 Normal Operating Conditions
The ophthalmic system shall be able to normally operate under the following conditions:
---Ambient temperature: 10 C ~ 30 C;
---Relative humidity: not greater than 70%;
---Atmospheric pressure: 86.0 kPa ~ 106.0 kPa;
---Power supply used: AC 220 V/(100 V ~ 240 V), 50 Hz/60 Hz;
---There is no strong electromagnetic field interference around:
---There is no obvious vibration or disturbing airflow around.
4.2 Therapeutic Laser
4.2.1 Laser wavelength
Laser wavelength is 1,064 nm 5 nm.
4.2.2 Laser mode
4.2.4 Terminal output energy of laser pulse / pulse train
4.2.4.1 Maximum terminal output energy
The manufacturer shall specify the nominal value of the maximum terminal output energy of
the laser pulse / pulse train in accordance with the laser pulse output mode, and its tolerance
shall not exceed 20%.
4.2.4.2 Terminal output energy regulation
The terminal output energy shall be adjustable. The manufacturer shall provide the range of
adjustment, and its tolerance shall not exceed 20%.
4.2.5 Reproducibility of laser output energy
It shall not exceed 20%.
4.2.6 Laser output beam convergence angle
The manufacturer shall specify the nominal value and tolerance (shall not exceed 20%) of the
laser output beam convergence angle, or the range of the beam convergence angle; the beam
convergence angle shall not be less than 14.
4.2.7 Focal spot diameter of laser output
The manufacturer shall specify the nominal value and tolerance (shall not exceed 20%) of the
focal spot diameter of laser output, or the range of focal spot diameter; the focal spot diameter
shall not be greater than 50 m.
4.3 Aiming System
4.3.1 Aiming light wavelength. The manufacturer shall specify the nominal value and tolerance
of the central wavelength of the aiming light, or the range of the central wavelength.
4.3.2 Aiming light power. It shall not be greater than 1 mW.
4.3.3 Coincidence of aiming light. The aiming light and the therapeutic laser shall coincide at
the focal plane.
4.4 Slit-lamp Microscope and Laser Radiation at the Eyepiece
4.4.1 The manufacturer shall specify the performance requirements of the slit-lamp microscope
in accordance with the applicable clauses of YY 0065-2007.
4.4.2 The laser radiation at the eyepiece of the slit-lamp microscope shall not exceed AEL value
of Class I in GB 7247.1-2001.
4.5 Appearance
4.5.1 The appearance shall be clean, uniform in color, and free from corrosion, coating peeling,
scars, scratches, deformation and other defects.
4.5.2 The characters and marks shall be clearly visible.
4.5.3 The control and adjustment mechanism shall be flexible and reliable, the fastening parts
shall not be loose, the button switch shall have a clear hand feeling, and the actions shall be
reliable.
4.6 Safety
The ophthalmic system shall comply with the requirements of GB 9706.1-2007, GB 9706.20-
2000 and GB 7247.1-2001.
The manufacturer shall respectively specify the safety characteristics (including insulation
diagrams) and applicable terms in accordance with GB 9706.1-2007, GB 9706.20-2000 and GB
7247.1-2001.
4.7 Environmental Adaptability
The environmental adaptability of the ophthalmic system shall comply with the requirements
of GB/T 14710-1993; the manufacturer shall provide the specific test conditions and inspection
items. The inspection items shall at least include the maximum terminal output energy in 4.2.4.1.
5 Test Methods
5.1 General
The test methods listed in this Chapter are recommended. If the same effect can be obtained,
other test methods are also allowed to be used.
5.2 Therapeutic Laser
5.2.1 Laser wavelength
Use a wavelength meter or a spectrometer to conduct the measurement, and calculate the error.
The results shall comply with the requirements of 4.2.1.
5.2.2 Laser mode
Use a laser beam analyzer to conduct the inspection. The results shall comply with the
requirements of 4.2.2.
5.2.3 Time characteristics of laser pulse output
The test method for the time characteristics is:
a) Use a photoelectric probe and an oscilloscope to carry out the test, and record the
5.3.2 Laser power of aiming light
Use a laser power meter to conduct the measurement, and the results shall com...
Share