1
/
의
8
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1090-2018 English PDF (YYT1090-2018)
YY/T 1090-2018 English PDF (YYT1090-2018)
정가
$100.00 USD
정가
할인가
$100.00 USD
단가
/
단위
배송료는 결제 시 계산됩니다.
픽업 사용 가능 여부를 로드할 수 없습니다.
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY/T 1090-2018
Historical versions: YY/T 1090-2018
Preview True-PDF (Reload/Scroll if blank)
YY/T 1090-2018: Ultrasonic physiotherapy equipment
YY/T 1090-2018
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.60
C 41
Replacing YY 1090-2009
Ultrasonic Physiotherapy Equipment
超声理疗设备
ISSUED ON: DECEMBER 20, 2018
IMPLEMENTED ON: JANUARY 1, 2020
Issued by: National Medical Products Administration
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative References ... 4
3 Terms and Definitions ... 4
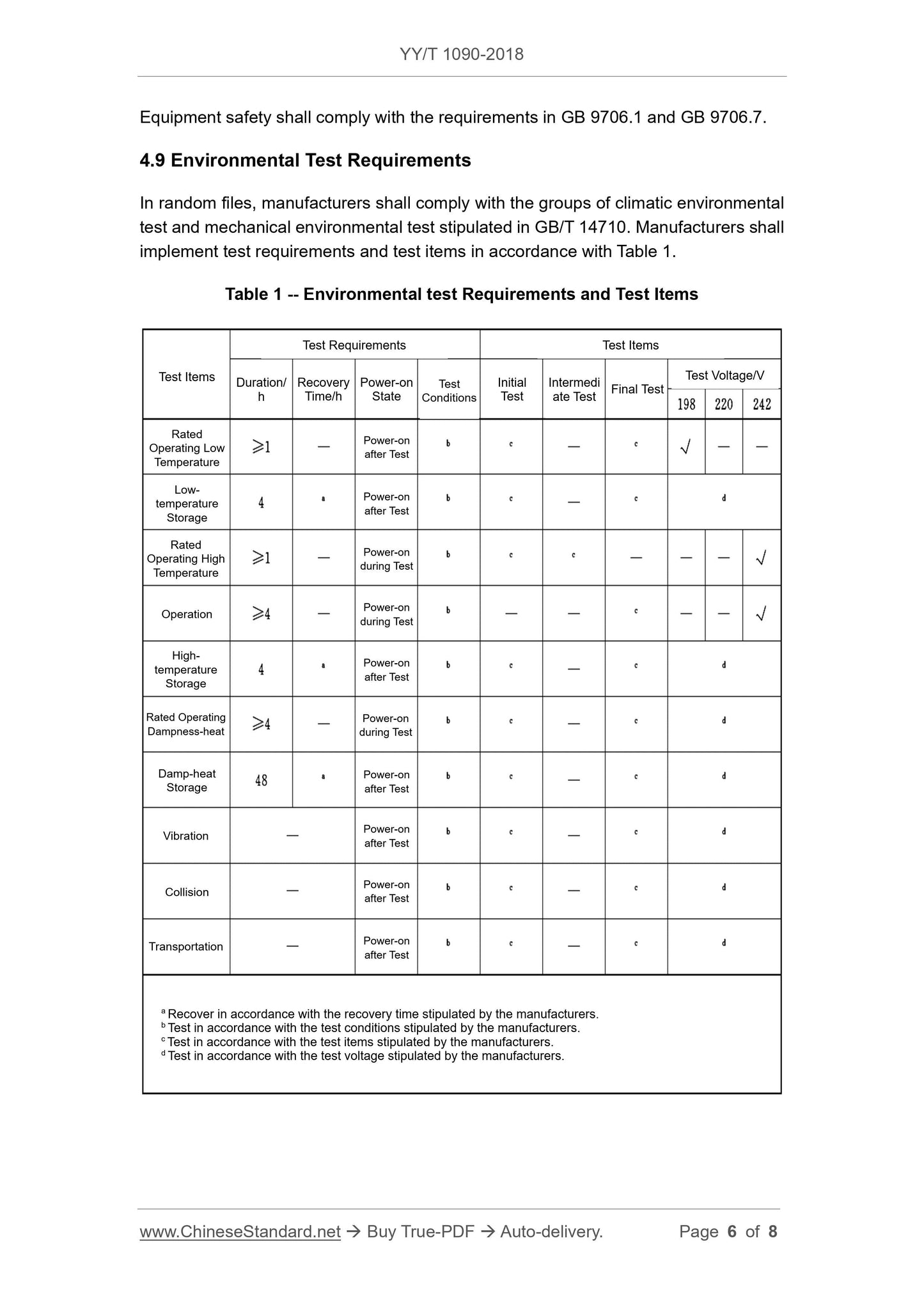
4 Requirements ... 5
5 Test Methods ... 7
6 Inspection Rules ... 8
Ultrasonic Physiotherapy Equipment
1 Scope
This Standard specifies the requirements, test methods and inspection rules of
ultrasonic physiotherapy equipment.
This Standard is applicable to ultrasonic physiotherapy equipment, which generates
continuous wave or quasi-continuous wave ultrasonic energy through planar circular
ultrasonic transducer within the frequency range of 0.5 MHz ~ 5 MHz (hereinafter
referred to as equipment). This Standard is not applicable to equipment whose
effective sound intensity is more than 3 W/cm2, or equipment which adopts focused
ultrasound.
2 Normative References
The following documents are indispensable to the application of this Standard. In terms
of references with a specified date, only versions with a specified date are applicable
to this Standard. In terms of references without a specified date, the latest version
(including all the modifications) of is applicable to this Standard.
GB 9706.1 Medical Electrical Equipment - Part 1: General Requirements for
Safety
GB 9706.7 Medical Electrical Equipment - Part 2-5: Particular Requirements for
the Safety of Ultrasonic Physiotherapy Equipment
GB/T 14710 Environmental Requirement and Test Methods for Medical Electrical
Equipment
YY/T 0750-2018 Ultrasonic - Physiotherapy Systems - Field Specifications and
Methods of Measurement in the Frequency Range 0.5 MHz to 5 MHz
YY/T 0865.1-2011 Ultrasonic - Hydrophone - Part 1: Measurement and
Characterization of Medical Ultrasonic Fields up to 40 MHz
3 Terms and Definitions
Terms and definitions defined in GB 9706.7 and YY/T 0750 are applicable to this
Standard.
5 Test Methods
5.1 Accuracy of Ultrasonic Output Power
Ultrasonic output power shall comply with the test methods stipulated in 7.2 and 7.3 in
YY/T 0750-2018.
The measurement of the rated ultrasonic output power and the rated ultrasonic time
maximum output power shall respectively be conducted under the condition of 90%,
100% and 110% rated power supply voltage. During the measurement, manual
adjustment of the equipment is no longer allowed. Take the most unfavorable value to
examine whether the measurement result complies with the requirements.
Respectively measure at 1/3 and 2/3 point (or nearby, or gear) of the ultrasonic output
power and the rated ultrasonic time maximum output power, so as to verify the
deviation between the indicated power and the actually measured value.
5.2 Effective Radiation Area
Comply with the test methods stipulated in 7.4 in YY/T 0750-2018. The deviation shall
be calculated in accordance with Formula (1).
5.3 Effective Sound Intensity
In accordance with the measured value of output power and effective radiation area,
calculate effective sound intensity.
5.4 Acoustic Operating Frequency
Comply with the test methods stipulated in 7.3 in YY/T 0865.1-2011 and YY/T 0750-
2018. The deviation shall be calculated in accordance with Formula (1).
5.5 Beam Non-uniformity Ratio
Comply with the test methods stipulated in 7.4 in YY/T 0750-2018.
5.6 Appearance and Structure
Conduct visual inspection on appearance and structure. It shall comply with the
requirements in 4.7.
5.7 Random Files
Examine random files. It shall comply with the requirements in 4.8.
Deviation
Indicated Value
Measured Value Indicated Value
Get QUOTATION in 1-minute: Click YY/T 1090-2018
Historical versions: YY/T 1090-2018
Preview True-PDF (Reload/Scroll if blank)
YY/T 1090-2018: Ultrasonic physiotherapy equipment
YY/T 1090-2018
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.60
C 41
Replacing YY 1090-2009
Ultrasonic Physiotherapy Equipment
超声理疗设备
ISSUED ON: DECEMBER 20, 2018
IMPLEMENTED ON: JANUARY 1, 2020
Issued by: National Medical Products Administration
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative References ... 4
3 Terms and Definitions ... 4
4 Requirements ... 5
5 Test Methods ... 7
6 Inspection Rules ... 8
Ultrasonic Physiotherapy Equipment
1 Scope
This Standard specifies the requirements, test methods and inspection rules of
ultrasonic physiotherapy equipment.
This Standard is applicable to ultrasonic physiotherapy equipment, which generates
continuous wave or quasi-continuous wave ultrasonic energy through planar circular
ultrasonic transducer within the frequency range of 0.5 MHz ~ 5 MHz (hereinafter
referred to as equipment). This Standard is not applicable to equipment whose
effective sound intensity is more than 3 W/cm2, or equipment which adopts focused
ultrasound.
2 Normative References
The following documents are indispensable to the application of this Standard. In terms
of references with a specified date, only versions with a specified date are applicable
to this Standard. In terms of references without a specified date, the latest version
(including all the modifications) of is applicable to this Standard.
GB 9706.1 Medical Electrical Equipment - Part 1: General Requirements for
Safety
GB 9706.7 Medical Electrical Equipment - Part 2-5: Particular Requirements for
the Safety of Ultrasonic Physiotherapy Equipment
GB/T 14710 Environmental Requirement and Test Methods for Medical Electrical
Equipment
YY/T 0750-2018 Ultrasonic - Physiotherapy Systems - Field Specifications and
Methods of Measurement in the Frequency Range 0.5 MHz to 5 MHz
YY/T 0865.1-2011 Ultrasonic - Hydrophone - Part 1: Measurement and
Characterization of Medical Ultrasonic Fields up to 40 MHz
3 Terms and Definitions
Terms and definitions defined in GB 9706.7 and YY/T 0750 are applicable to this
Standard.
5 Test Methods
5.1 Accuracy of Ultrasonic Output Power
Ultrasonic output power shall comply with the test methods stipulated in 7.2 and 7.3 in
YY/T 0750-2018.
The measurement of the rated ultrasonic output power and the rated ultrasonic time
maximum output power shall respectively be conducted under the condition of 90%,
100% and 110% rated power supply voltage. During the measurement, manual
adjustment of the equipment is no longer allowed. Take the most unfavorable value to
examine whether the measurement result complies with the requirements.
Respectively measure at 1/3 and 2/3 point (or nearby, or gear) of the ultrasonic output
power and the rated ultrasonic time maximum output power, so as to verify the
deviation between the indicated power and the actually measured value.
5.2 Effective Radiation Area
Comply with the test methods stipulated in 7.4 in YY/T 0750-2018. The deviation shall
be calculated in accordance with Formula (1).
5.3 Effective Sound Intensity
In accordance with the measured value of output power and effective radiation area,
calculate effective sound intensity.
5.4 Acoustic Operating Frequency
Comply with the test methods stipulated in 7.3 in YY/T 0865.1-2011 and YY/T 0750-
2018. The deviation shall be calculated in accordance with Formula (1).
5.5 Beam Non-uniformity Ratio
Comply with the test methods stipulated in 7.4 in YY/T 0750-2018.
5.6 Appearance and Structure
Conduct visual inspection on appearance and structure. It shall comply with the
requirements in 4.7.
5.7 Random Files
Examine random files. It shall comply with the requirements in 4.8.
Deviation
Indicated Value
Measured Value Indicated Value
Share