1
/
의
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1470-2016 English PDF (YYT1470-2016)
YY/T 1470-2016 English PDF (YYT1470-2016)
정가
$130.00 USD
정가
할인가
$130.00 USD
단가
/
단위
배송료는 결제 시 계산됩니다.
픽업 사용 가능 여부를 로드할 수 없습니다.
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY/T 1470-2016
Historical versions: YY/T 1470-2016
Preview True-PDF (Reload/Scroll if blank)
YY/T 1470-2016: Disposable cut of the umbilical cord
YY/T 1470-2016
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.30
C 36
Disposable cut of the umbilical cord
ISSUED ON. JANUARY 26, 2016
IMPLEMENTED ON. JANUARY 1, 2017
Issued by. China Food and Drug Administration
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative References ... 4
3 Structural styles ... 5
4 Requirements ... 6
5 Test methods ... 8
6 Inspection rules ... 10
7 Marking and operation instructions ... 11
8 Packaging, transportation and storage ... 13
Foreword
This Standard was drafted in accordance with the rules given in GB/T 1.1-2009.
Attention is drawn to the possibility that some of the elements of this document may
be the subject of patent rights. The issuer of this document shall not be held
responsible for identifying any or all such patent rights.
This Standard was proposed by and shall be under the jurisdiction of Technical
Committee 169 on Family Planning Instruments of Standardization Administration of
China (SAC/TC 169).
The drafting organizations of this Standard. Jiangsu Sinolinks Mecial Innovation Inc.,
Shanghai Testing and Inspection Institute for Medical Devices, Shanghai Hengyi
Weighing and Instrument Co., Ltd.
The main drafters of this Standard. Tian Runting, Yao Tianping, Weng Binghao, Zou
Bing, Jiang Songbo.
Disposable cut of the umbilical cord
1 Scope
This Standard specifies the structural styles, requirements, test methods, inspection
rules, marking and operation instructions, packaging, transportation and storage for
disposable cut of the umbilical cord.
This Standard applies to disposable cut of the umbilical cord. The product is used to
clamp and cut the newborn umbilical cord during obstetrical delivery.
2 Normative References
The following referenced documents are indispensable for the application of this
document. For dated references, only the edition dated applies to this document. For
undated references, the latest edition of the referenced documents (including all
amendments) applies to This Standard.
GB/T 191, Packaging – Pictorial marking for handling of goods
GB/T 2828.1, Sampling procedures for inspection by attributes - Part 1. Sampling
schemes indexed by acceptance quality limit(AQL) for lot-by-lot inspection
GB/T 2829, Sampling procedures and tables for periodic inspection by attributes
(Apply to inspection of process stability)
GB 3280-2015, Cold rolled stainless steel plate, sheet and strip
GB/T 4340.1-2009, Metallic materials - Vickers hardness test - Part 1. Test method
GB/T 9969, General principles for preparation of instructions for use of industrial
products
GB/T 14233.1-2008, Test methods for infusion, transfusion, injection equipment for
medical use - Part 1. Chemical analysis methods
GB/T 16886.1, Biological evaluation of medical devices - Part 1. Evaluation and
testing
GB/T 16886.5-2003, Biological evaluation of medical devices - Part 5. Test for in
vitro cytotoxicity
GB/T 16886.10-2005, Biological evaluation of medical devices - Part 10. Tests for
irritation and delayed-type hypersensitivity
YY/T 0031-2008, Silicone tubes and elastomeric parts for infusion and transfusion
YY/T 0149-2006, Medical instruments of stainless steel - Test methods of corrosion
resistance
YY/T 0171, Surgical instruments - packaging, marking and instructions
Pharmacopoeia of the People's Republic of China, Fourth Part (2015 Edition)
3 Structural styles
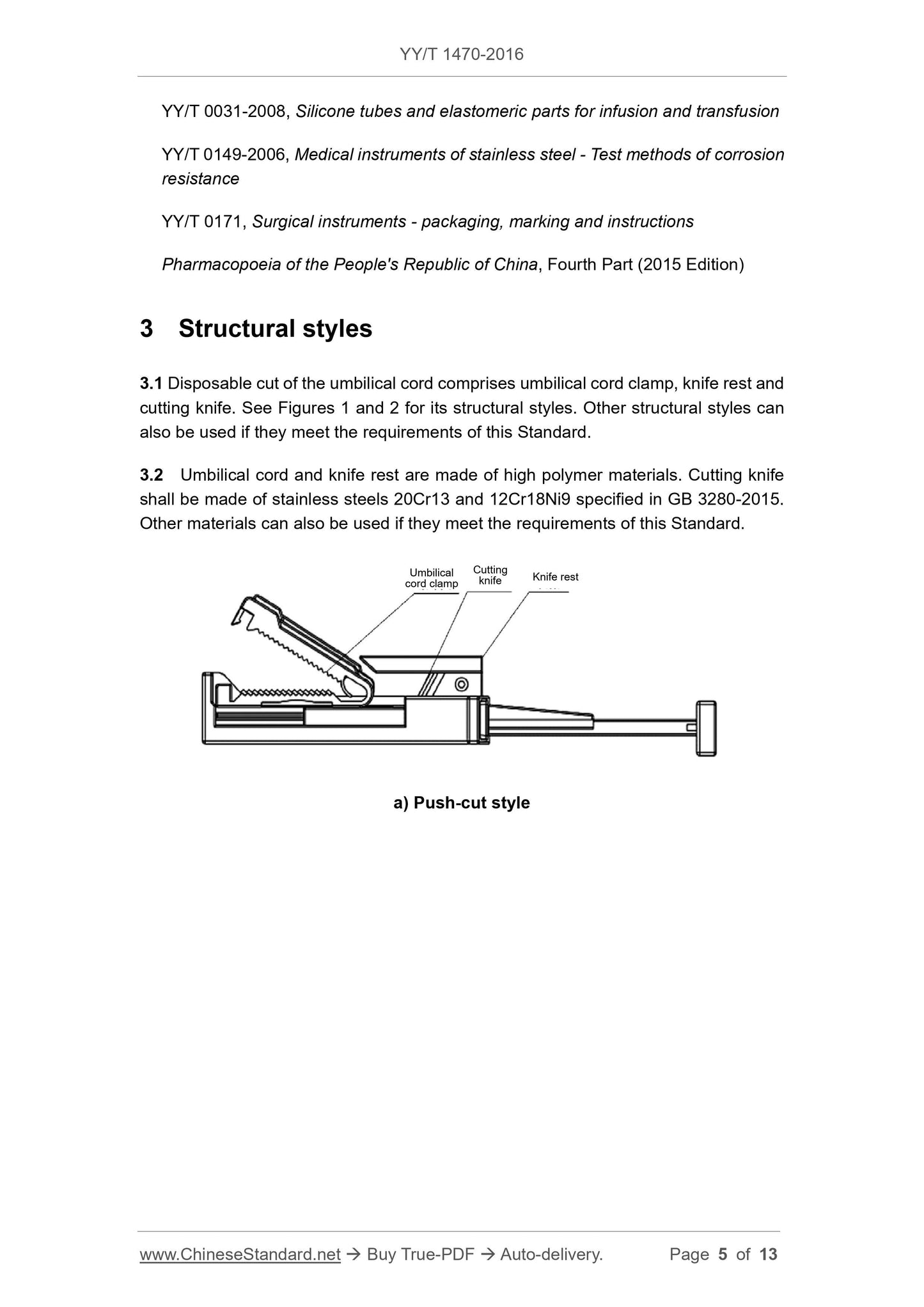
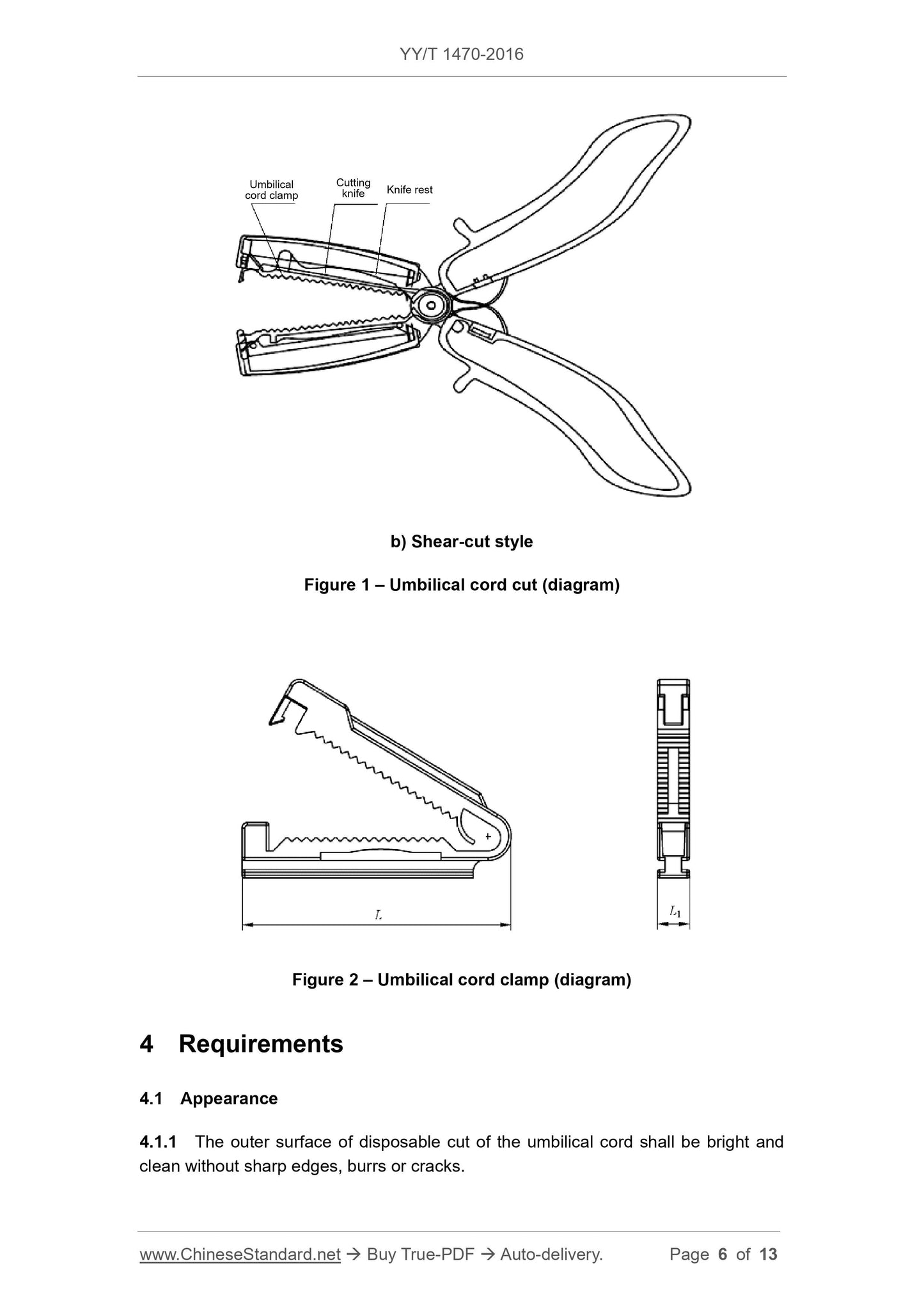
3.1 Disposable cut of the umbilical cord comprises umbilical cord clamp, knife rest and
cutting knife. See Figures 1 and 2 for its structural styles. Other structural styles can
also be used if they meet the requirements of this Standard.
3.2 Umbilical cord and knife rest are made of high polymer materials. Cutting knife
shall be made of stainless steels 20Cr13 and 12Cr18Ni9 specified in GB 3280-2015.
Other materials can also be used if they meet the requirements of this Standard.
a) Push-cut style
Umbilical cord clamp
Cutting knife Knife rest
b) Shear-cut style
Figure 1 – Umbilical cord cut (diagram)
Figure 2 – Umbilical cord clamp (diagram)
4 Requirements
4.1 Appearance
4.1.1 The outer surface of disposable cut of the umbilical cord shall be bright and
clean without sharp edges, burrs or cracks.
Umbilical
cord clamp
Cutting
knife Knife rest
4.1.2 The surface of cutting knife shall be smooth without oil contamination, rust or
broken edge.
4.2 Dimensions
The dimensions of umbilical cord clamp shall meet the requirements of Table 1.
Table 1 – Basic dimensions and tolerances Dimensions in mm
Dimension of umbilical cord clamp Dimension Tolerance
L 50 ~ 60 ± 2
L1 5 ~ 7 ± 0.5
4.3 Cutting blade
4.3.1 Surface roughness
The surface roughness of cutting blade shall not be greater than 0.8 μm.
4.3.2 Hardness
The hardness of cutting blade shall not be less than 377 HV0.2.
4.3.3 Corrosion resistance
Cutting blade shall be well corrosion resistant. Its corrosion resistance shall be as
specified for grade b of the boiling test method of YY/T 0149-2006.
4.4 Service performance
4.4.1 Cutting performance
Disposable cut of the umbilical cord shall have good cutting performance.
4.4.2 Clamping performance
Disposable cut of the umbilical cord shall have good clamping performance, which
shall be capable of bearing a 15 N static load.
4.4.3 Fitting performance
The push frame of disposable cut of the umbilical cord shall have good fitting
performance, which shall be free from falling-off, jamming etc. during cutting.
4.5 Sterility
For the sterilization through a confirmed sterilization process, the sterilized disposable
cut of the umbilical cord shall be sterile.
4.6 Ethylene oxide residue
If disposable cut of the umbilical cord is sterilized using ethylene oxide, the residue of
ethylene oxide shall not be greater than 10 μg/g.
4.7 Biological evaluation
4.7.1 In vitro cytotoxicity
The cytotoxic reaction of disposable cut of the umbilical cord shall not be greater than
grade 2.
4.7.2 Delayed-type hypersensitivity
Disposable cut of the umbilical cord shall not cause delayed-type hypersensitivity.
4.7.3 Intradermal reaction
Compare test sample of disposable cut of the umbilical cord and solvent; and the
average difference between scores shall not be greater than 1.0.
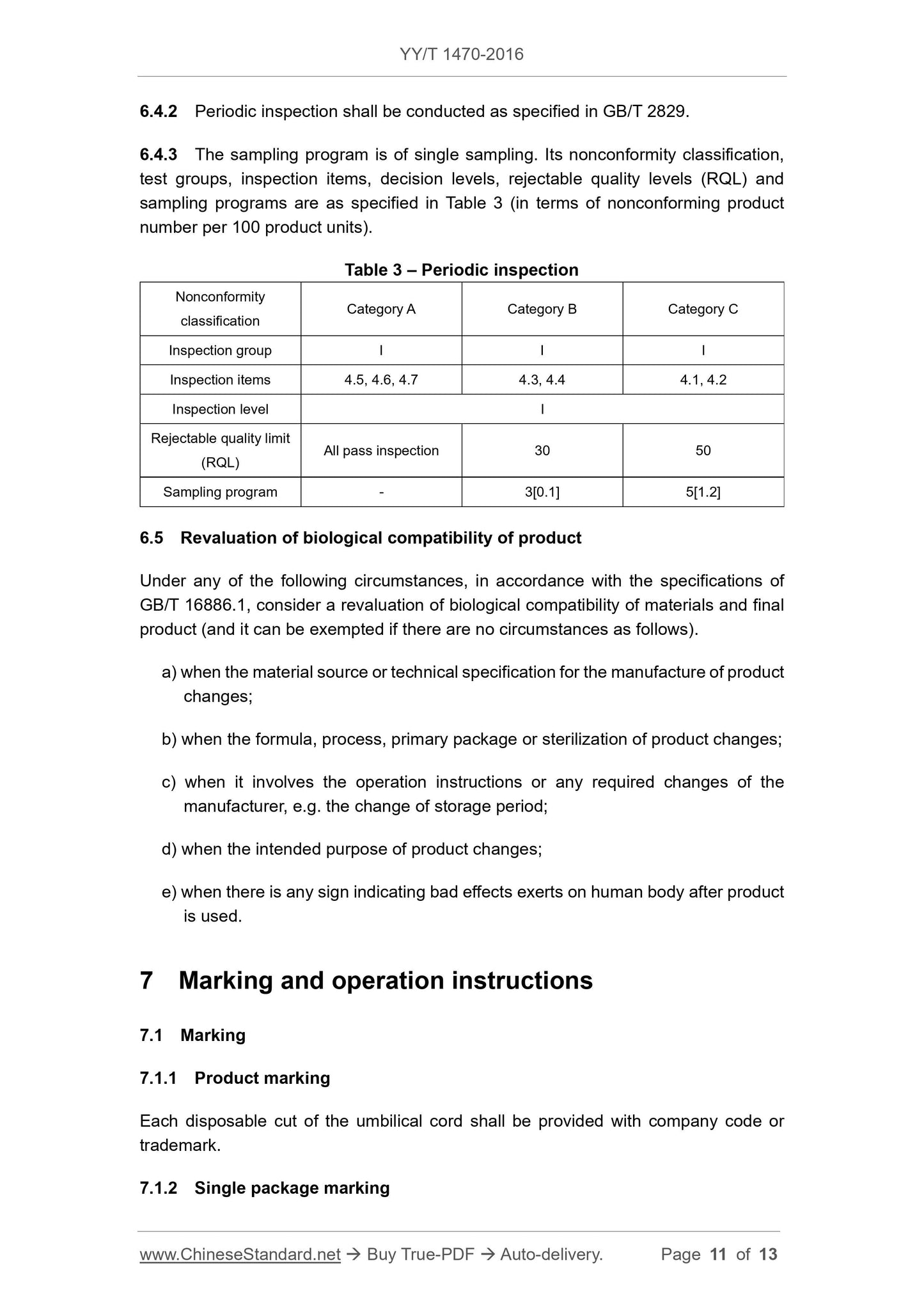
5 Test methods
5.1 Appearance
5.1.1 Use normal or corrected visual acuity to check and use hand to feel. It shall be
as specified in 4.1.1.
5.1.2 Use normal or corrected visual acuity to check. It shall be as specified in 4.1.2.
5.2 Dimensions
Use general measuring tools for measurement. They shall be as specified in 4.2.
5.3 Cutting blade
5.3.1 Surface roughness
Use a surface roughness comparison specimen or the electrical measuring method for
measurement; use the electrical measuring method in arbitration; and it shall be as
specified in 4.3.1.
5.3.2 Hardness
Conduct test using the method specified in GB/T 4340.1-2009. It shall be as specified
in 4.3.2.
5.3.3 Corrosion resistance
Conduct test using the boiling method specified in YY/T 0149-2006. It shall be as
specified in 4.3.3.
5.4 Service performance
5.4.1 Cutting performance
Use the infusion tube 5 mm × 7 mm specified in Table 1 of YY/T 0031-2008; and
simulate the movement of shearing (cutting). The cut surface of the infusion tube shall
be smooth without adhesion; and the cutting blad...
Get QUOTATION in 1-minute: Click YY/T 1470-2016
Historical versions: YY/T 1470-2016
Preview True-PDF (Reload/Scroll if blank)
YY/T 1470-2016: Disposable cut of the umbilical cord
YY/T 1470-2016
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.30
C 36
Disposable cut of the umbilical cord
ISSUED ON. JANUARY 26, 2016
IMPLEMENTED ON. JANUARY 1, 2017
Issued by. China Food and Drug Administration
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative References ... 4
3 Structural styles ... 5
4 Requirements ... 6
5 Test methods ... 8
6 Inspection rules ... 10
7 Marking and operation instructions ... 11
8 Packaging, transportation and storage ... 13
Foreword
This Standard was drafted in accordance with the rules given in GB/T 1.1-2009.
Attention is drawn to the possibility that some of the elements of this document may
be the subject of patent rights. The issuer of this document shall not be held
responsible for identifying any or all such patent rights.
This Standard was proposed by and shall be under the jurisdiction of Technical
Committee 169 on Family Planning Instruments of Standardization Administration of
China (SAC/TC 169).
The drafting organizations of this Standard. Jiangsu Sinolinks Mecial Innovation Inc.,
Shanghai Testing and Inspection Institute for Medical Devices, Shanghai Hengyi
Weighing and Instrument Co., Ltd.
The main drafters of this Standard. Tian Runting, Yao Tianping, Weng Binghao, Zou
Bing, Jiang Songbo.
Disposable cut of the umbilical cord
1 Scope
This Standard specifies the structural styles, requirements, test methods, inspection
rules, marking and operation instructions, packaging, transportation and storage for
disposable cut of the umbilical cord.
This Standard applies to disposable cut of the umbilical cord. The product is used to
clamp and cut the newborn umbilical cord during obstetrical delivery.
2 Normative References
The following referenced documents are indispensable for the application of this
document. For dated references, only the edition dated applies to this document. For
undated references, the latest edition of the referenced documents (including all
amendments) applies to This Standard.
GB/T 191, Packaging – Pictorial marking for handling of goods
GB/T 2828.1, Sampling procedures for inspection by attributes - Part 1. Sampling
schemes indexed by acceptance quality limit(AQL) for lot-by-lot inspection
GB/T 2829, Sampling procedures and tables for periodic inspection by attributes
(Apply to inspection of process stability)
GB 3280-2015, Cold rolled stainless steel plate, sheet and strip
GB/T 4340.1-2009, Metallic materials - Vickers hardness test - Part 1. Test method
GB/T 9969, General principles for preparation of instructions for use of industrial
products
GB/T 14233.1-2008, Test methods for infusion, transfusion, injection equipment for
medical use - Part 1. Chemical analysis methods
GB/T 16886.1, Biological evaluation of medical devices - Part 1. Evaluation and
testing
GB/T 16886.5-2003, Biological evaluation of medical devices - Part 5. Test for in
vitro cytotoxicity
GB/T 16886.10-2005, Biological evaluation of medical devices - Part 10. Tests for
irritation and delayed-type hypersensitivity
YY/T 0031-2008, Silicone tubes and elastomeric parts for infusion and transfusion
YY/T 0149-2006, Medical instruments of stainless steel - Test methods of corrosion
resistance
YY/T 0171, Surgical instruments - packaging, marking and instructions
Pharmacopoeia of the People's Republic of China, Fourth Part (2015 Edition)
3 Structural styles
3.1 Disposable cut of the umbilical cord comprises umbilical cord clamp, knife rest and
cutting knife. See Figures 1 and 2 for its structural styles. Other structural styles can
also be used if they meet the requirements of this Standard.
3.2 Umbilical cord and knife rest are made of high polymer materials. Cutting knife
shall be made of stainless steels 20Cr13 and 12Cr18Ni9 specified in GB 3280-2015.
Other materials can also be used if they meet the requirements of this Standard.
a) Push-cut style
Umbilical cord clamp
Cutting knife Knife rest
b) Shear-cut style
Figure 1 – Umbilical cord cut (diagram)
Figure 2 – Umbilical cord clamp (diagram)
4 Requirements
4.1 Appearance
4.1.1 The outer surface of disposable cut of the umbilical cord shall be bright and
clean without sharp edges, burrs or cracks.
Umbilical
cord clamp
Cutting
knife Knife rest
4.1.2 The surface of cutting knife shall be smooth without oil contamination, rust or
broken edge.
4.2 Dimensions
The dimensions of umbilical cord clamp shall meet the requirements of Table 1.
Table 1 – Basic dimensions and tolerances Dimensions in mm
Dimension of umbilical cord clamp Dimension Tolerance
L 50 ~ 60 ± 2
L1 5 ~ 7 ± 0.5
4.3 Cutting blade
4.3.1 Surface roughness
The surface roughness of cutting blade shall not be greater than 0.8 μm.
4.3.2 Hardness
The hardness of cutting blade shall not be less than 377 HV0.2.
4.3.3 Corrosion resistance
Cutting blade shall be well corrosion resistant. Its corrosion resistance shall be as
specified for grade b of the boiling test method of YY/T 0149-2006.
4.4 Service performance
4.4.1 Cutting performance
Disposable cut of the umbilical cord shall have good cutting performance.
4.4.2 Clamping performance
Disposable cut of the umbilical cord shall have good clamping performance, which
shall be capable of bearing a 15 N static load.
4.4.3 Fitting performance
The push frame of disposable cut of the umbilical cord shall have good fitting
performance, which shall be free from falling-off, jamming etc. during cutting.
4.5 Sterility
For the sterilization through a confirmed sterilization process, the sterilized disposable
cut of the umbilical cord shall be sterile.
4.6 Ethylene oxide residue
If disposable cut of the umbilical cord is sterilized using ethylene oxide, the residue of
ethylene oxide shall not be greater than 10 μg/g.
4.7 Biological evaluation
4.7.1 In vitro cytotoxicity
The cytotoxic reaction of disposable cut of the umbilical cord shall not be greater than
grade 2.
4.7.2 Delayed-type hypersensitivity
Disposable cut of the umbilical cord shall not cause delayed-type hypersensitivity.
4.7.3 Intradermal reaction
Compare test sample of disposable cut of the umbilical cord and solvent; and the
average difference between scores shall not be greater than 1.0.
5 Test methods
5.1 Appearance
5.1.1 Use normal or corrected visual acuity to check and use hand to feel. It shall be
as specified in 4.1.1.
5.1.2 Use normal or corrected visual acuity to check. It shall be as specified in 4.1.2.
5.2 Dimensions
Use general measuring tools for measurement. They shall be as specified in 4.2.
5.3 Cutting blade
5.3.1 Surface roughness
Use a surface roughness comparison specimen or the electrical measuring method for
measurement; use the electrical measuring method in arbitration; and it shall be as
specified in 4.3.1.
5.3.2 Hardness
Conduct test using the method specified in GB/T 4340.1-2009. It shall be as specified
in 4.3.2.
5.3.3 Corrosion resistance
Conduct test using the boiling method specified in YY/T 0149-2006. It shall be as
specified in 4.3.3.
5.4 Service performance
5.4.1 Cutting performance
Use the infusion tube 5 mm × 7 mm specified in Table 1 of YY/T 0031-2008; and
simulate the movement of shearing (cutting). The cut surface of the infusion tube shall
be smooth without adhesion; and the cutting blad...
Share