1
/
의
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1879-2022 English PDF (YYT1879-2022)
YY/T 1879-2022 English PDF (YYT1879-2022)
정가
$140.00 USD
정가
할인가
$140.00 USD
단가
/
단위
배송료는 결제 시 계산됩니다.
픽업 사용 가능 여부를 로드할 수 없습니다.
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY/T 1879-2022
Historical versions: YY/T 1879-2022
Preview True-PDF (Reload/Scroll if blank)
YY/T 1879-2022: Creation and placement of unique device identifier
YY/T 1879-2022
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040
CCS C 30
Creation and placement of unique device identifier
ISSUED ON: AUGUST 17, 2022
IMPLEMENTED ON: DECEMBER 01, 2022
Issued by: National Medical Products Administration
Table of Contents
Foreword ... 3
Introduction ... 4
1 Scope ... 5
2 Normative references ... 5
3 Terms, definitions and abbreviations ... 5
4 Requirements for UDI creation ... 6
5 Requirements for UDI placement links ... 7
6 Requirements for the creation and placement of UDIs for certain types of medical
devices ... 10
Bibliography ... 12
Creation and placement of unique device identifier
1 Scope
This document specifies the requirements for creation and placement of unique device
identifier.
This document is applicable to the implementation and application of the unique device
identifier by all relevant parties.
2 Normative references
The following referenced documents are indispensable for the application of this
document. For dated references, only the edition cited applies. For undated references,
the latest edition of the referenced document (including any amendments) applies.
YY/T 1681, Basic terms of unique device identification system
ISO 15223-1:2021, Medical devices - Symbols to be used with information to be
supplied by the manufacturer - Part 1: General requirements
3 Terms, definitions and abbreviations
3.1 Terms and definitions
For the purposes of this document, the terms and definitions defined in YY/T 1681
apply.
3.2 Abbreviations
The following abbreviations apply to this document.
AIDC: automatic identification and data capture
HRI: human readable information
UDI: unique device identifier
UDID: unique device identification database
UDI-DI: device identifier
UDI-PI: production identifier
UoU UDI-DI: unit of use device identifier
4 Requirements for UDI creation
The requirements followed in the UDI creation process are as follows.
a) UDI shall include UDI-DI and UDI-PI.
b) The UDI shall be created according to the coding rules of the selected code-issuing
institution. If it is otherwise stipulated in national regulations and standards,
follow its regulations.
c) A UDI shall be assigned to the smallest sales unit of a medical device. Higher-
level packages (excluding shipping packages) shall have their own UDIs.
d) A different UDI-DI shall be assigned to each product packaging level of a medical
device. See Table B.1 in YY/T 1630-2018. Maintain association relationship in
UDID.
e) When the smallest sales unit contains multiple identical units of use, the UoU
UDI-DI shall be assigned to the unit of use and stored in the UDID in order to
associate the use of the device with the patient.
f) UDI-DI shall have stability. If the basic characteristics of the medical device have
not changed, the UDI-DI shall remain unchanged. However, a new UDI-DI needs
to be assigned if there are changes that may lead to misidentification of the
medical device and/or unclear traceability, such as the number of products in the
package, whether it is sterile packaged and/or whether it is marked for single use
Wait for changes.
g) The composition of UDI-PI shall be consistent with the content of the label. For
example, when the medical device label contains one or more of the production
batch number, serial number, production date and expiration date of the medical
device product, it is recommended to be used as the UDI-PI component. Its
content shall be consistent with the corresponding information on the label. If the
expression format of the date is involved, it shall meet the requirements of the
coding rules of the selected code-issuing agency.
h) For medical devices controlled by batch production, considering the application
scenario, if a single product needs to be identified, it is advisable to add a serial
number on the basis of the combined use of UDI-DI and the production batch
number, OR add additional data separators according to the selected code issuer
encoding rules.
6 Requirements for the creation and placement of UDIs for
certain types of medical devices
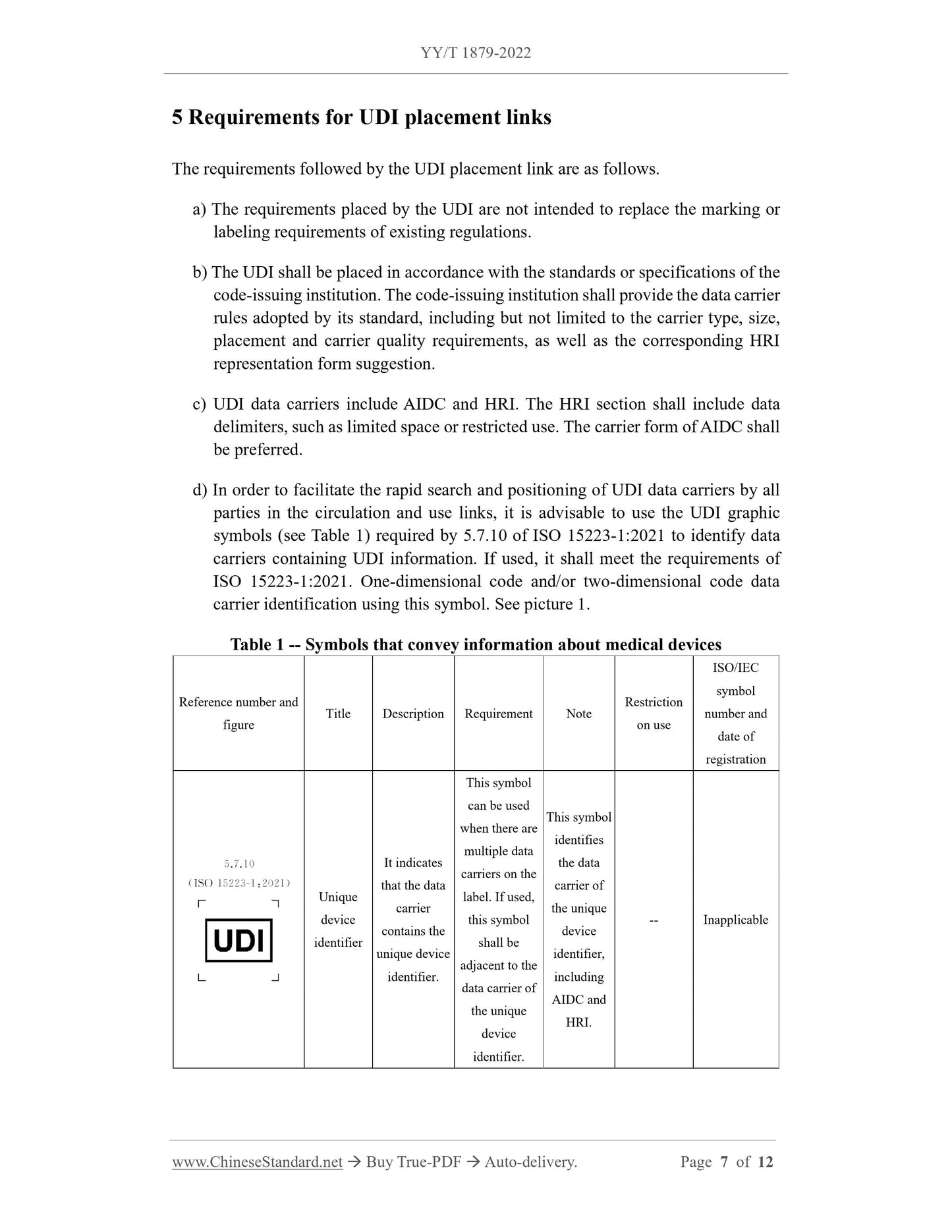
6.1 Medical device package
The requirements for the medical device package to be followed in the creation and
placement of UDI are as follows:
a) The medical device package sold separately shall have an independent UDI.
b) If the medical devices in the medical device package are sold separately, they shall
have an independent UDI.
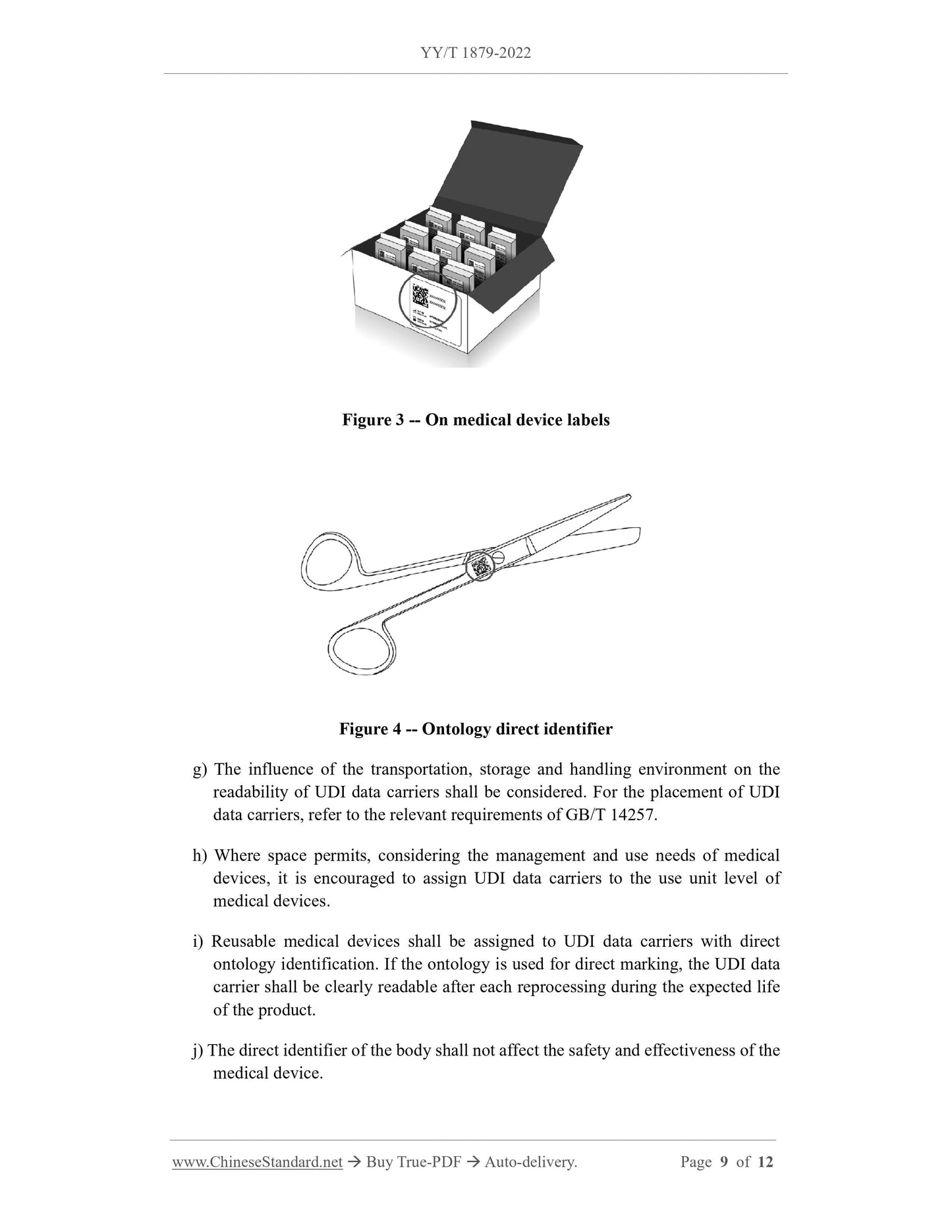
c) The single-use medical device in the medical device package does not need to
have an independent UDI if it is not used under conditions other than the medical
device package.
6.2 Independent software
The independent software follows the following requirements in the process of UDI
creation and placement.
a) The independent software shall assign UDIs at the product level.
b) The complete version of software is an important tool to realize the traceability of
independent software, which shall be reflected in UDI-PI.
c) A new UDI-DI shall be allocated for a major software update of independent
software (that is, affecting product safety or effectiveness). Only minor software
updates shall assign new UDI-PI instead of UDI-DI.
d) Normally, the software version can be represented by the data separator of the
production batch number. If the code-issuing organization assigns a specific data
separator to the software version, it can also be specified accordingly.
e) When delivering stand-alone software via physical media such as CD or DVD,
each product packaging level shall carry a full UDI for AIDC and HRI. The UDI
assigned on the physical medium containing the software shall be consistent with
the UDI assigned to the system software.
f) It shall be in human readable plain text format. Provide UDIs on user-friendly
interfaces (for example on "file or start screen").
g) For the independent software without a user interface, it shall be possible to send
UDI through an application programming interface (API).
Get QUOTATION in 1-minute: Click YY/T 1879-2022
Historical versions: YY/T 1879-2022
Preview True-PDF (Reload/Scroll if blank)
YY/T 1879-2022: Creation and placement of unique device identifier
YY/T 1879-2022
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040
CCS C 30
Creation and placement of unique device identifier
ISSUED ON: AUGUST 17, 2022
IMPLEMENTED ON: DECEMBER 01, 2022
Issued by: National Medical Products Administration
Table of Contents
Foreword ... 3
Introduction ... 4
1 Scope ... 5
2 Normative references ... 5
3 Terms, definitions and abbreviations ... 5
4 Requirements for UDI creation ... 6
5 Requirements for UDI placement links ... 7
6 Requirements for the creation and placement of UDIs for certain types of medical
devices ... 10
Bibliography ... 12
Creation and placement of unique device identifier
1 Scope
This document specifies the requirements for creation and placement of unique device
identifier.
This document is applicable to the implementation and application of the unique device
identifier by all relevant parties.
2 Normative references
The following referenced documents are indispensable for the application of this
document. For dated references, only the edition cited applies. For undated references,
the latest edition of the referenced document (including any amendments) applies.
YY/T 1681, Basic terms of unique device identification system
ISO 15223-1:2021, Medical devices - Symbols to be used with information to be
supplied by the manufacturer - Part 1: General requirements
3 Terms, definitions and abbreviations
3.1 Terms and definitions
For the purposes of this document, the terms and definitions defined in YY/T 1681
apply.
3.2 Abbreviations
The following abbreviations apply to this document.
AIDC: automatic identification and data capture
HRI: human readable information
UDI: unique device identifier
UDID: unique device identification database
UDI-DI: device identifier
UDI-PI: production identifier
UoU UDI-DI: unit of use device identifier
4 Requirements for UDI creation
The requirements followed in the UDI creation process are as follows.
a) UDI shall include UDI-DI and UDI-PI.
b) The UDI shall be created according to the coding rules of the selected code-issuing
institution. If it is otherwise stipulated in national regulations and standards,
follow its regulations.
c) A UDI shall be assigned to the smallest sales unit of a medical device. Higher-
level packages (excluding shipping packages) shall have their own UDIs.
d) A different UDI-DI shall be assigned to each product packaging level of a medical
device. See Table B.1 in YY/T 1630-2018. Maintain association relationship in
UDID.
e) When the smallest sales unit contains multiple identical units of use, the UoU
UDI-DI shall be assigned to the unit of use and stored in the UDID in order to
associate the use of the device with the patient.
f) UDI-DI shall have stability. If the basic characteristics of the medical device have
not changed, the UDI-DI shall remain unchanged. However, a new UDI-DI needs
to be assigned if there are changes that may lead to misidentification of the
medical device and/or unclear traceability, such as the number of products in the
package, whether it is sterile packaged and/or whether it is marked for single use
Wait for changes.
g) The composition of UDI-PI shall be consistent with the content of the label. For
example, when the medical device label contains one or more of the production
batch number, serial number, production date and expiration date of the medical
device product, it is recommended to be used as the UDI-PI component. Its
content shall be consistent with the corresponding information on the label. If the
expression format of the date is involved, it shall meet the requirements of the
coding rules of the selected code-issuing agency.
h) For medical devices controlled by batch production, considering the application
scenario, if a single product needs to be identified, it is advisable to add a serial
number on the basis of the combined use of UDI-DI and the production batch
number, OR add additional data separators according to the selected code issuer
encoding rules.
6 Requirements for the creation and placement of UDIs for
certain types of medical devices
6.1 Medical device package
The requirements for the medical device package to be followed in the creation and
placement of UDI are as follows:
a) The medical device package sold separately shall have an independent UDI.
b) If the medical devices in the medical device package are sold separately, they shall
have an independent UDI.
c) The single-use medical device in the medical device package does not need to
have an independent UDI if it is not used under conditions other than the medical
device package.
6.2 Independent software
The independent software follows the following requirements in the process of UDI
creation and placement.
a) The independent software shall assign UDIs at the product level.
b) The complete version of software is an important tool to realize the traceability of
independent software, which shall be reflected in UDI-PI.
c) A new UDI-DI shall be allocated for a major software update of independent
software (that is, affecting product safety or effectiveness). Only minor software
updates shall assign new UDI-PI instead of UDI-DI.
d) Normally, the software version can be represented by the data separator of the
production batch number. If the code-issuing organization assigns a specific data
separator to the software version, it can also be specified accordingly.
e) When delivering stand-alone software via physical media such as CD or DVD,
each product packaging level shall carry a full UDI for AIDC and HRI. The UDI
assigned on the physical medium containing the software shall be consistent with
the UDI assigned to the system software.
f) It shall be in human readable plain text format. Provide UDIs on user-friendly
interfaces (for example on "file or start screen").
g) For the independent software without a user interface, it shall be possible to send
UDI through an application programming interface (API).
Share