1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
GB 14880-2012 English PDF (GB14880-2012)
GB 14880-2012 English PDF (GB14880-2012)
Regular price
$145.00 USD
Regular price
Sale price
$145.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click GB 14880-2012
Historical versions: GB 14880-2012
Preview True-PDF (Reload/Scroll if blank)
GB 14880-2012: National food safety standard -- Standard for the use of nutritional fortification substances in foods
GB 14880-2012
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
GB 14880-2012
National Food Safety Standard for the Use of
Nutritional Fortification Substances in Foods
ISSUED ON: MARCH 15, 2012
IMPLEMENTED ON: JANUARY 01, 2013
Issued by: Ministry of Health of the People's Republic of China
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Terms and Definitions ... 4
3 Fundamental Purposes of Nutritional Fortification ... 4
4 Requirements of Using Nutritional Fortification Substances ... 5
5 Selection Requirements of Fortification Food Categories ... 5
6 Application Rules of Nutritional Fortification Substances ... 5
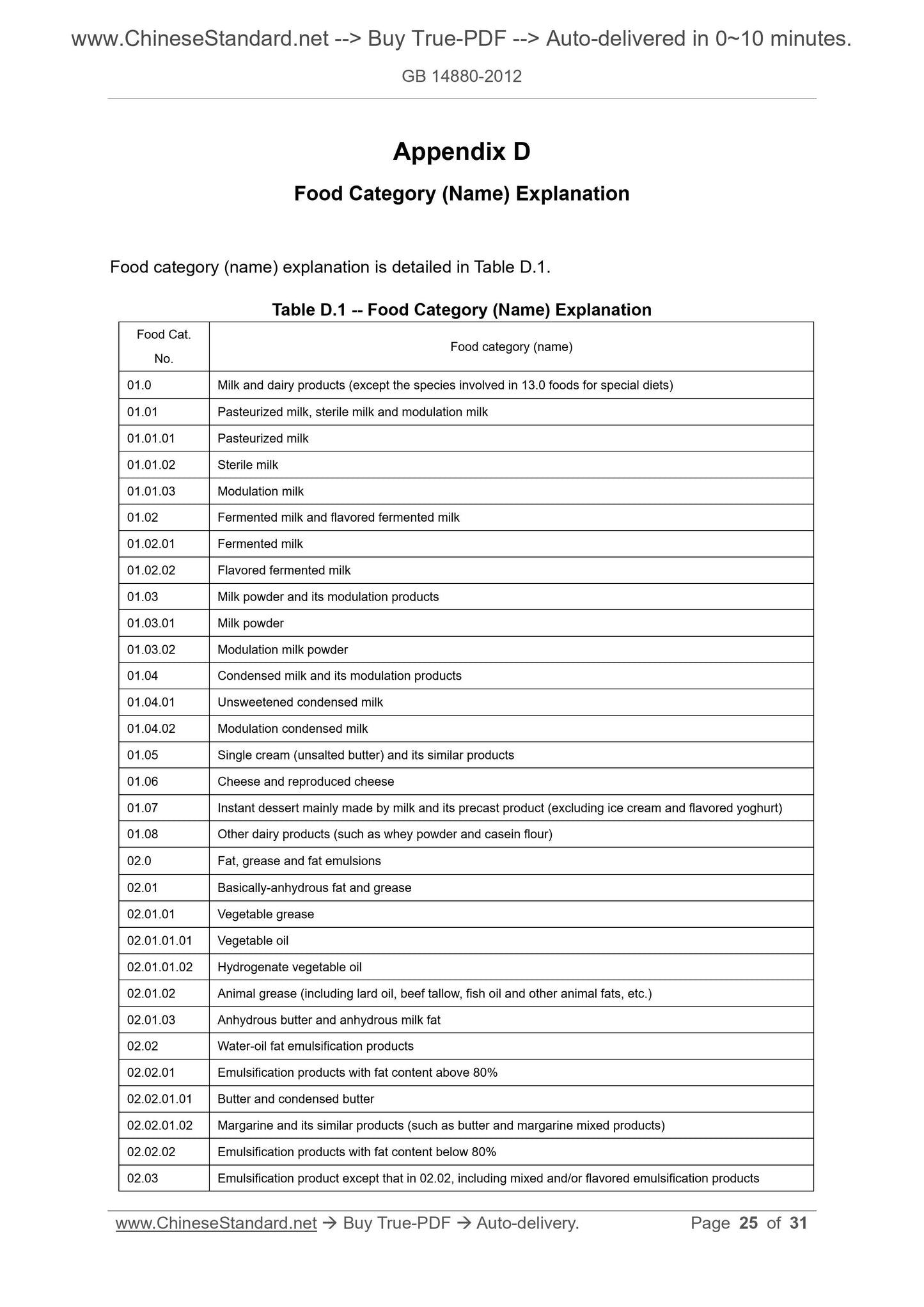
7 Food Category (Name) Description ... 6
8 Quality Standard of Nutritional Fortification Substances ... 6
Appendix A Application Rules of Nutritional Fortification Substances in Foods ... 7
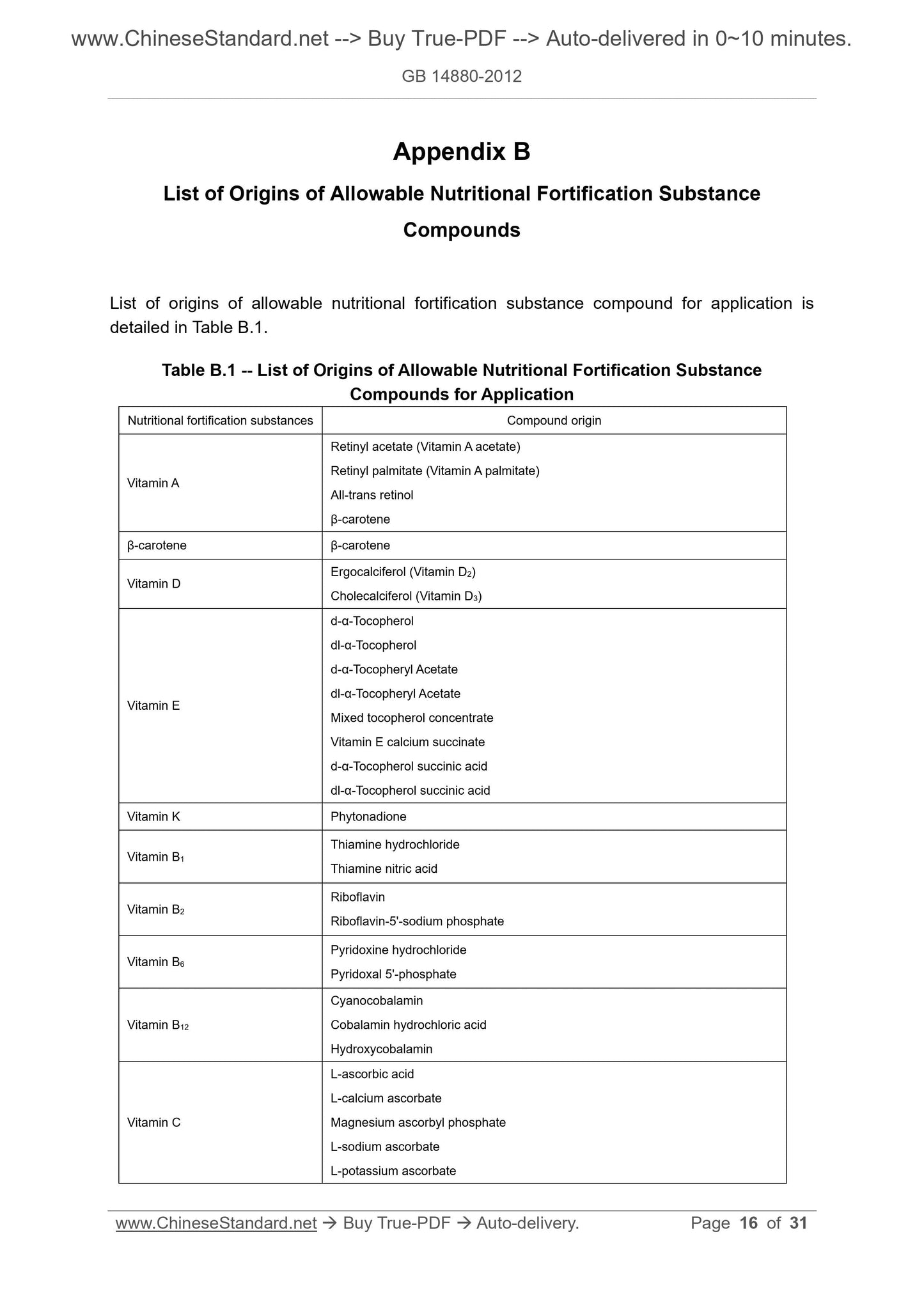
Appendix B List of Origins of Allowable Nutritional Fortification Substance
Compounds ... 16
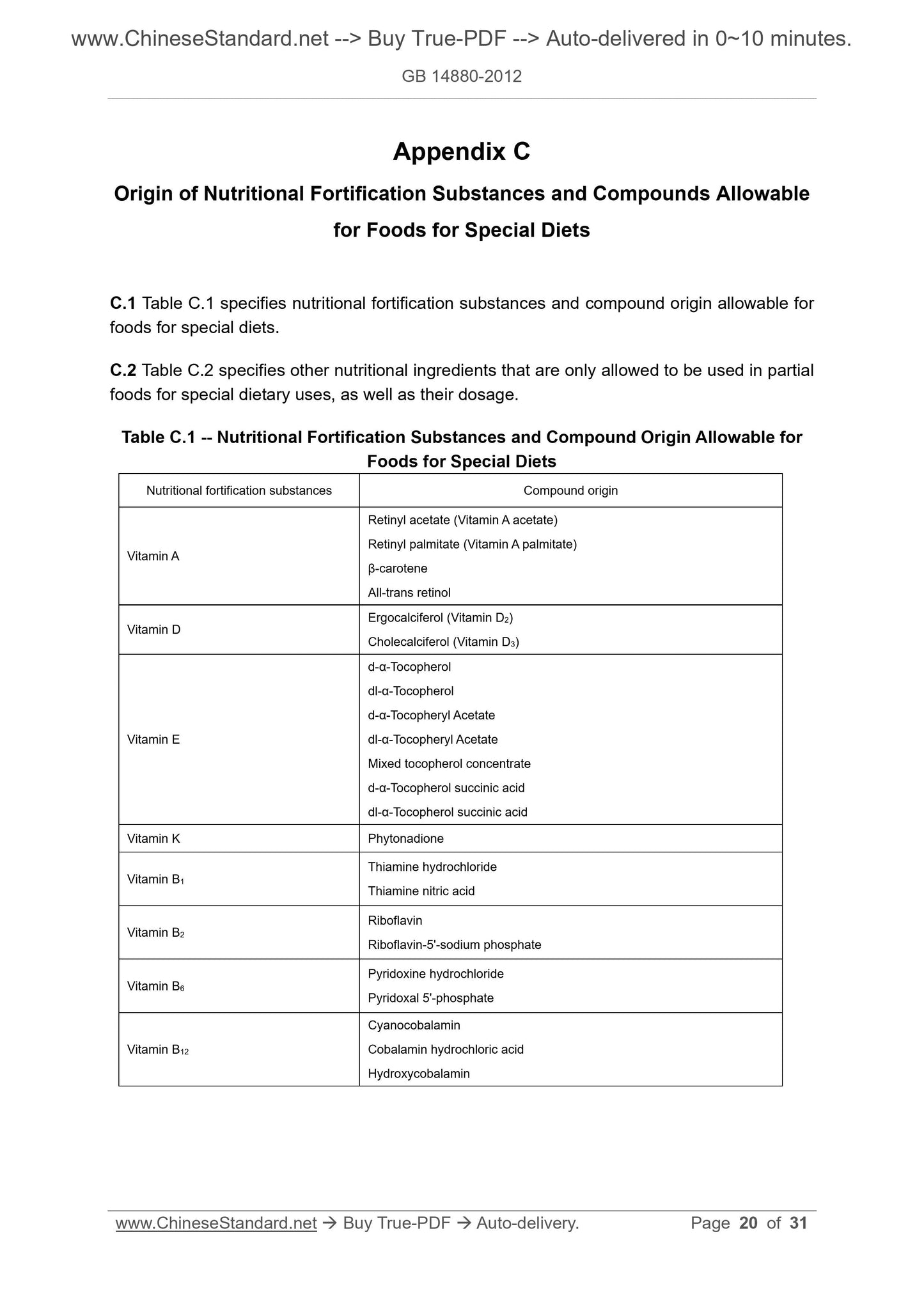
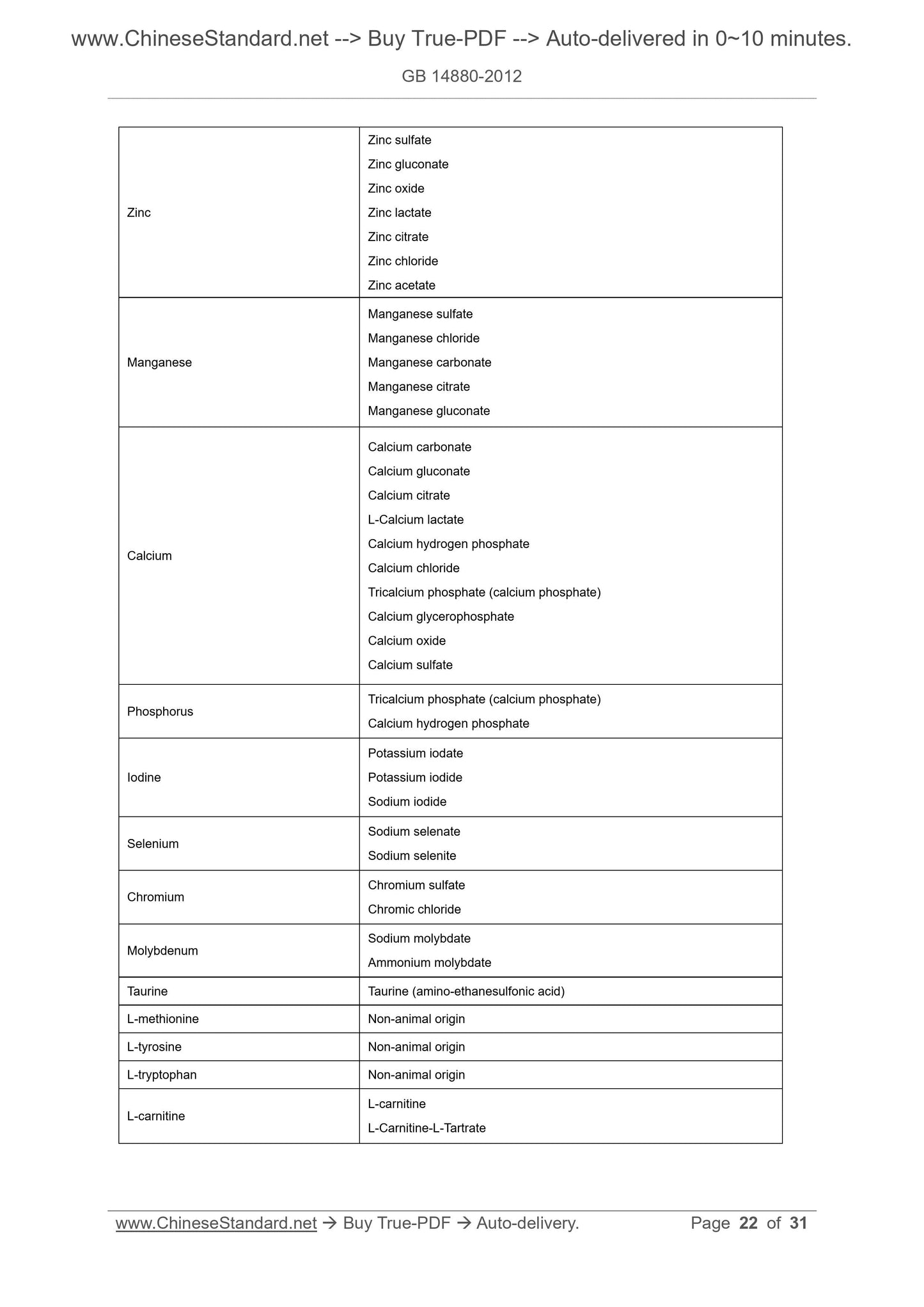
Appendix C Origin of Nutritional Fortification Substances and Compounds Allowable
for Foods for Special Diets ... 20
Appendix D Food Category (Name) Explanation ... 25
National Food Safety Standard for the Use of
Nutritional Fortification Substances in Foods
1 Scope
This Standard specifies the fundamental purposes of nutritional fortification in foods, the
requirements of using nutritional fortification substances, the selection requirements for
fortifiable food categories and application requirements for nutritional fortification substances.
This Standard is applicable to the application of nutritional fortification substances in foods,
unless otherwise stated in national laws and regulations and/or standards.
2 Terms and Definitions
2.1 Nutritional fortification substances
The natural or artificially-synthesized nutrients and other nutritional ingredients added to foods
in order to increase the nutritional ingredient (value) in foods.
2.2 Nutrient
The substances which have specific physiological effects in foods and are required and able
to maintain the growth, development, activity, reproduction, and normal metabolism, including
protein, fat, carbohydrate, mineral substances and vitamin, etc.
2.3 Other nutritional ingredients
Other food compositions which have nutrition and/or physiological functions except nutrients
2.4 Foods for special diets
Food made by special process or formula in order to meet special physical/psychological
conditions and/or meet special diet demands under the conditions of diseases or disorder. The
contents of nutrients and/or other nutritional ingredients in these foods are obviously different
from comparable ordinary foods.
3 Fundamental Purposes of Nutritional Fortification
3.1 Make up the nutrient loss caused during normal process and storage of foods.
3.2 In a given territorial scope, where the intake of some nutrients for a large scale of crowd is
low or lacked, the caused poor health effects may be improved by fortification.
3.3 The nutrient intake level for some crowd is low or lacked because of the dietary habit and/or
other causes, the caused poor health effects may be improved by fortification.
3.4 Replenish and adjust the contents of nutrients and/or other nutritional ingredients in foods
for special diets.
4 Requirements of Using Nutritional Fortification
Substances
4.1 Utilization of nutritional fortification substances shall not result in intake excess or
imbalance of nutrients or other nutritional ingredients, neither the metabolism abnormality of
any nutrient and other nutritional ingredients.
4.2 Utilization of nutritional fortification substances shall not encourage and lead the food
consumption pattern against the national nutrition policies.
4.3 The quality of nutritional fortification substances added to foods shall be able to be kept
stable under specific conditions of storage, transportation and eating.
4.4 The nutritional fortification substances added to foods shall not result in obvious bad
changes of general characteristics of foods, such as color, luster, flavor, smell and cooking
characteristics.
4.5 The content or effect of a certain nutritional ingredient shall not be exaggerated by the
application of nutritional fortification substances to mislead and cheat consumers.
5 Selection Requirements of Fortification Food Categories
5.1 Foods consumed universally by target population and obtained easily shall be selected for
fortification.
5.2 The consumption of foods as the fortification carriers shall be relatively stable.
5.3 The foods which are advocated to reduce the eating amount in the diet guideline of China
should not be used as the fortification carriers.
6 Application Rules of Nutritional Fortification Substances
6.1 Application scope and application amount of nutritional fortification substances in foods
shall meet the requirements of Appendix A; the origin of the allowable utilization compound
Appendix A
Application Rules of Nutritional Fortification Substances in Foods
Application rules of nutritional fortification substances in foods are detailed in Table A.1.
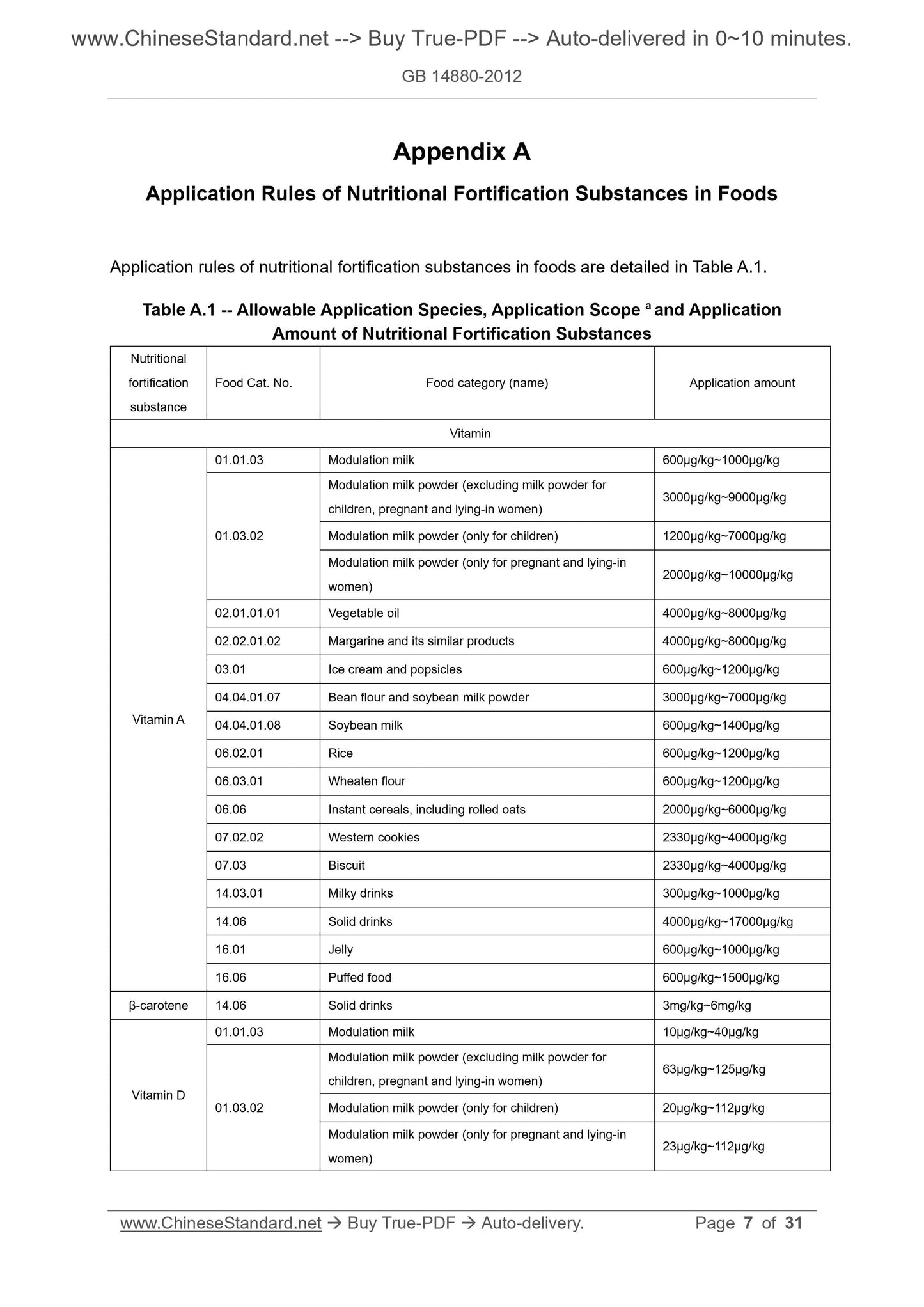
Table A.1 -- Allowable Application Species, Application Scope a and Application
Amount of Nutritional Fortification Substances
Nutritional
fortification
substance
Food Cat. No. Food category (name) Application amount
Vitamin
Vitamin A
01.01.03 Modulation milk 600μg/kg~1000μg/kg
01.03.02
Modulation milk powder (excluding milk powder for
children, pregnant and lying-in women) 3000μg/kg~9000μg/kg
Modulation milk powder (only for children) 1200μg/kg~7000μg/kg
Modulation milk powder (only for pregnant and lying-in
women) 2000μg/kg~10000μg/kg
02.01.01.01 Vegetable oil 4000μg/kg~8000μg/kg
02.02.01.02 Margarine and its similar products 4000μg/kg~8000μg/kg
03.01 Ice cream and popsicles 600μg/kg~1200μg/kg
04.04.01.07 Bean flour and soybean milk powder 3000μg/kg~7000μg/kg
04.04.01.08 Soybean milk 600μg/kg~1400μg/kg
06.02.01 Rice 600μg/kg~1200μg/kg
06.03.01 Wheaten flour 600μg/kg~1200μg/kg
06.06 Instant cereals, including rolled oats 2000μg/kg~6000μg/kg
07.02.02 Western cookies 2330μg/kg~4000μg/kg
07.03 Biscuit 2330μg/kg~4000μg/kg
14.03.01 Milky drinks 300μg/kg~1000μg/kg
14.06 Solid drinks 4000μg/kg~17000μg/kg
16.01 Jelly 600μg/kg~1000μg/kg
16.06 Puffed food 600μg/kg~1500μg/kg
β-carotene 14.06 Solid drinks 3mg/kg~6mg/kg
Vitamin D
01.01.03 Modulation milk 10μg/kg~40μg/kg
01.03.02
Modulation milk powder (excluding milk powder for
children, pregnant and lying-in women) 63μg/kg~125μg/kg
Modulation milk powder (only for children) 20μg/kg~112μg/kg
Modulation milk powder (only for pregnant and lying-in
women) 23μg/kg~112μg/kg
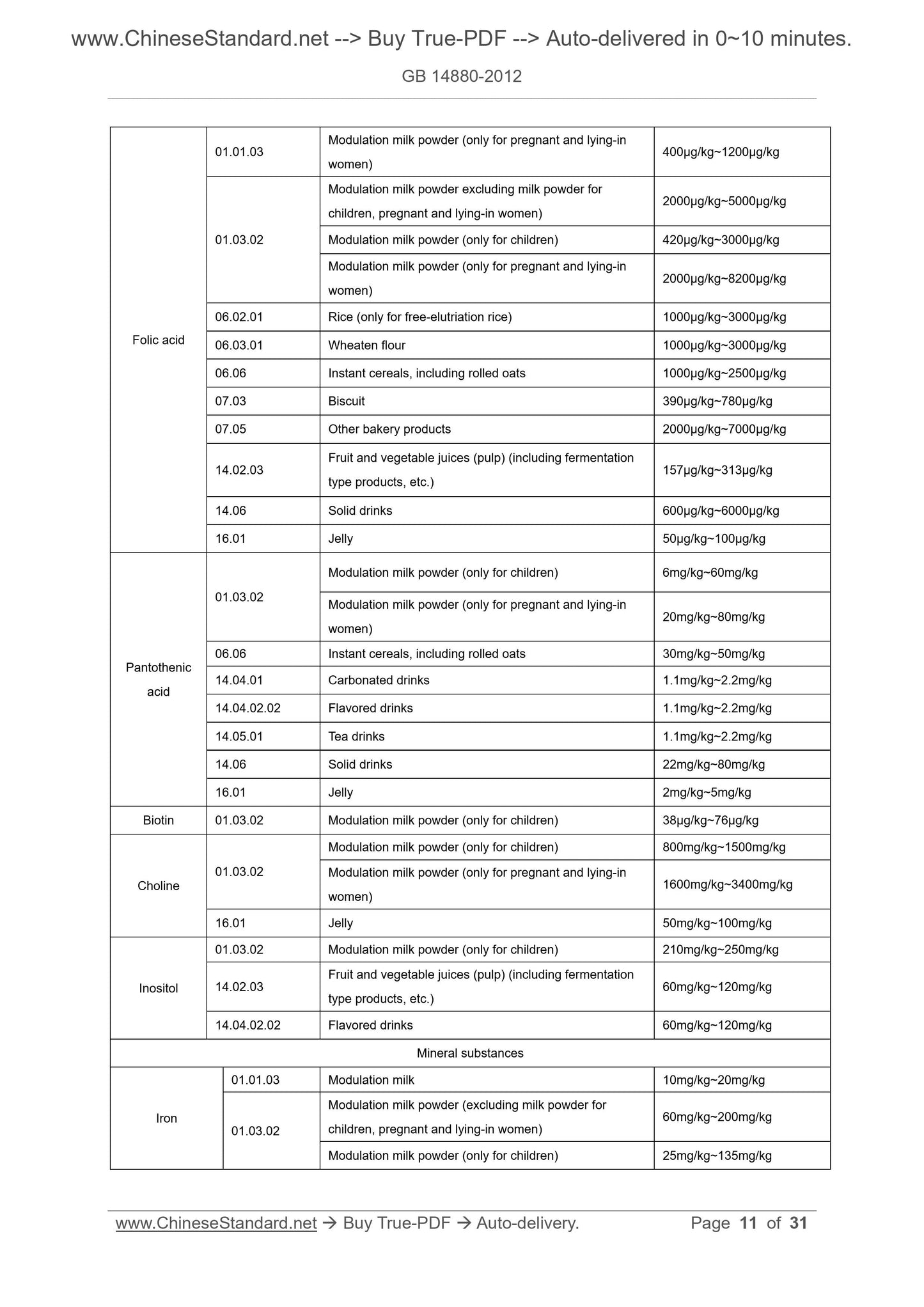
Folic acid
01.01.03 Modulation milk powder (only for pregnant and lying-in women) 400μg/kg~1200μg/kg
01.03.02
Modulation milk powder excluding milk powder for
children, pregnant and lying-in women) 2000μg/kg~5000μg/kg
Modulation milk powder (only for children) 420μg/kg~3000μg/kg
Modulation milk powder (only for pregnant and lying-in
women) 2000μg/kg~8200μg/kg
06.02.01 Rice (only for free-elutriation rice) 1000μg/kg~3000μg/kg
06.03.01 Wheaten flour 1000μg/kg~3000μg/kg
06.06 Instant cereals, including rolled oats 1000μg/kg~2500μg/kg
07.03 Biscuit 390μg/kg~780μg/kg
07.05 Other bakery products 2000μg/kg~7000μg/kg
14.02.03 Fruit and vegetable juices (pulp) (including fermentation type products, etc.) 157μg/kg~313μg/kg
14.06 Solid drinks 600μg/kg~6000μg/kg
16.01 Jel...
Get QUOTATION in 1-minute: Click GB 14880-2012
Historical versions: GB 14880-2012
Preview True-PDF (Reload/Scroll if blank)
GB 14880-2012: National food safety standard -- Standard for the use of nutritional fortification substances in foods
GB 14880-2012
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
GB 14880-2012
National Food Safety Standard for the Use of
Nutritional Fortification Substances in Foods
ISSUED ON: MARCH 15, 2012
IMPLEMENTED ON: JANUARY 01, 2013
Issued by: Ministry of Health of the People's Republic of China
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Terms and Definitions ... 4
3 Fundamental Purposes of Nutritional Fortification ... 4
4 Requirements of Using Nutritional Fortification Substances ... 5
5 Selection Requirements of Fortification Food Categories ... 5
6 Application Rules of Nutritional Fortification Substances ... 5
7 Food Category (Name) Description ... 6
8 Quality Standard of Nutritional Fortification Substances ... 6
Appendix A Application Rules of Nutritional Fortification Substances in Foods ... 7
Appendix B List of Origins of Allowable Nutritional Fortification Substance
Compounds ... 16
Appendix C Origin of Nutritional Fortification Substances and Compounds Allowable
for Foods for Special Diets ... 20
Appendix D Food Category (Name) Explanation ... 25
National Food Safety Standard for the Use of
Nutritional Fortification Substances in Foods
1 Scope
This Standard specifies the fundamental purposes of nutritional fortification in foods, the
requirements of using nutritional fortification substances, the selection requirements for
fortifiable food categories and application requirements for nutritional fortification substances.
This Standard is applicable to the application of nutritional fortification substances in foods,
unless otherwise stated in national laws and regulations and/or standards.
2 Terms and Definitions
2.1 Nutritional fortification substances
The natural or artificially-synthesized nutrients and other nutritional ingredients added to foods
in order to increase the nutritional ingredient (value) in foods.
2.2 Nutrient
The substances which have specific physiological effects in foods and are required and able
to maintain the growth, development, activity, reproduction, and normal metabolism, including
protein, fat, carbohydrate, mineral substances and vitamin, etc.
2.3 Other nutritional ingredients
Other food compositions which have nutrition and/or physiological functions except nutrients
2.4 Foods for special diets
Food made by special process or formula in order to meet special physical/psychological
conditions and/or meet special diet demands under the conditions of diseases or disorder. The
contents of nutrients and/or other nutritional ingredients in these foods are obviously different
from comparable ordinary foods.
3 Fundamental Purposes of Nutritional Fortification
3.1 Make up the nutrient loss caused during normal process and storage of foods.
3.2 In a given territorial scope, where the intake of some nutrients for a large scale of crowd is
low or lacked, the caused poor health effects may be improved by fortification.
3.3 The nutrient intake level for some crowd is low or lacked because of the dietary habit and/or
other causes, the caused poor health effects may be improved by fortification.
3.4 Replenish and adjust the contents of nutrients and/or other nutritional ingredients in foods
for special diets.
4 Requirements of Using Nutritional Fortification
Substances
4.1 Utilization of nutritional fortification substances shall not result in intake excess or
imbalance of nutrients or other nutritional ingredients, neither the metabolism abnormality of
any nutrient and other nutritional ingredients.
4.2 Utilization of nutritional fortification substances shall not encourage and lead the food
consumption pattern against the national nutrition policies.
4.3 The quality of nutritional fortification substances added to foods shall be able to be kept
stable under specific conditions of storage, transportation and eating.
4.4 The nutritional fortification substances added to foods shall not result in obvious bad
changes of general characteristics of foods, such as color, luster, flavor, smell and cooking
characteristics.
4.5 The content or effect of a certain nutritional ingredient shall not be exaggerated by the
application of nutritional fortification substances to mislead and cheat consumers.
5 Selection Requirements of Fortification Food Categories
5.1 Foods consumed universally by target population and obtained easily shall be selected for
fortification.
5.2 The consumption of foods as the fortification carriers shall be relatively stable.
5.3 The foods which are advocated to reduce the eating amount in the diet guideline of China
should not be used as the fortification carriers.
6 Application Rules of Nutritional Fortification Substances
6.1 Application scope and application amount of nutritional fortification substances in foods
shall meet the requirements of Appendix A; the origin of the allowable utilization compound
Appendix A
Application Rules of Nutritional Fortification Substances in Foods
Application rules of nutritional fortification substances in foods are detailed in Table A.1.
Table A.1 -- Allowable Application Species, Application Scope a and Application
Amount of Nutritional Fortification Substances
Nutritional
fortification
substance
Food Cat. No. Food category (name) Application amount
Vitamin
Vitamin A
01.01.03 Modulation milk 600μg/kg~1000μg/kg
01.03.02
Modulation milk powder (excluding milk powder for
children, pregnant and lying-in women) 3000μg/kg~9000μg/kg
Modulation milk powder (only for children) 1200μg/kg~7000μg/kg
Modulation milk powder (only for pregnant and lying-in
women) 2000μg/kg~10000μg/kg
02.01.01.01 Vegetable oil 4000μg/kg~8000μg/kg
02.02.01.02 Margarine and its similar products 4000μg/kg~8000μg/kg
03.01 Ice cream and popsicles 600μg/kg~1200μg/kg
04.04.01.07 Bean flour and soybean milk powder 3000μg/kg~7000μg/kg
04.04.01.08 Soybean milk 600μg/kg~1400μg/kg
06.02.01 Rice 600μg/kg~1200μg/kg
06.03.01 Wheaten flour 600μg/kg~1200μg/kg
06.06 Instant cereals, including rolled oats 2000μg/kg~6000μg/kg
07.02.02 Western cookies 2330μg/kg~4000μg/kg
07.03 Biscuit 2330μg/kg~4000μg/kg
14.03.01 Milky drinks 300μg/kg~1000μg/kg
14.06 Solid drinks 4000μg/kg~17000μg/kg
16.01 Jelly 600μg/kg~1000μg/kg
16.06 Puffed food 600μg/kg~1500μg/kg
β-carotene 14.06 Solid drinks 3mg/kg~6mg/kg
Vitamin D
01.01.03 Modulation milk 10μg/kg~40μg/kg
01.03.02
Modulation milk powder (excluding milk powder for
children, pregnant and lying-in women) 63μg/kg~125μg/kg
Modulation milk powder (only for children) 20μg/kg~112μg/kg
Modulation milk powder (only for pregnant and lying-in
women) 23μg/kg~112μg/kg
Folic acid
01.01.03 Modulation milk powder (only for pregnant and lying-in women) 400μg/kg~1200μg/kg
01.03.02
Modulation milk powder excluding milk powder for
children, pregnant and lying-in women) 2000μg/kg~5000μg/kg
Modulation milk powder (only for children) 420μg/kg~3000μg/kg
Modulation milk powder (only for pregnant and lying-in
women) 2000μg/kg~8200μg/kg
06.02.01 Rice (only for free-elutriation rice) 1000μg/kg~3000μg/kg
06.03.01 Wheaten flour 1000μg/kg~3000μg/kg
06.06 Instant cereals, including rolled oats 1000μg/kg~2500μg/kg
07.03 Biscuit 390μg/kg~780μg/kg
07.05 Other bakery products 2000μg/kg~7000μg/kg
14.02.03 Fruit and vegetable juices (pulp) (including fermentation type products, etc.) 157μg/kg~313μg/kg
14.06 Solid drinks 600μg/kg~6000μg/kg
16.01 Jel...
Share