1
/
of
8
PayPal, credit cards. Download editable-PDF and invoice in 1 second!
NY/T 939-2016 English PDF (NY/T939-2016)
NY/T 939-2016 English PDF (NY/T939-2016)
Regular price
$85.00 USD
Regular price
Sale price
$85.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click NY/T 939-2016
Historical versions: NY/T 939-2016
Preview True-PDF (Reload/Scroll if blank)
NY/T 939-2016: Identification of reconstituted milk in pasteurized and UHT milk
NY/T 939-2016
AGRICULTURE INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 67.050
X 04
Replacing NY/T 939-2005
Identification of Reconstituted
Milk in Pasteurized and UHT Milk
ISSUED ON: MARCH 23, 2016
IMPLEMENTED ON: APRIL 01, 2016
Issued by: Ministry of Agriculture of PRC
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative References ... 4
3 Terms and Definitions ... 4
4 Test Methods ... 5
5 Identification of Reconstituted Milk ... 16
Appendix A (Informative) Furosine Liquid Chromatogram ... 18
Identification of Reconstituted
Milk in Pasteurized and UHT Milk
1 Scope
This Standard specifies the identification method of reconstituted milk in pasteurized
and UHT milk.
This Standard is applicable to the pasteurized and UHT milk.
2 Normative References
The following documents are essential to the application of this document. For the
dated documents, only the versions with the dates indicated are applicable to this
document; for the undated documents, only the latest version (including all the
amendments) are applicable to this document.
GB 5009.5 Determination of Protein in Foods
GB/T 6682 Water for Laboratory Use – Specifications
GB/T 10111 Generation of Random Numbers and Procedures Applied to Sampling
Inspection for Product Quality
3 Terms and Definitions
For the purposes of this document, the following terms and definitions apply.
3.1 Raw milk
The normal milk extruded from the breasts of the healthy dairy animals, it meets the
relevant national requirements and has no ingredient changes.
3.2 Reconstituted milk
The milk obtained by mixing a dried or concentrated dairy product with water in
proportion.
3.3 Heat treatment
4.1.1 Principle
Hydrolyze the specimen by hydrochloric acid, then determine its protein content; after
diluting, the hydrolysate shall be analyzed by high performance liquid chromatography
(HPLC) or ultra-high-performance liquid chromatography (UPLC) under ultraviolet
(wavelength 280nm) detector and quantify by external standard method.
4.1.2 Reagents and materials
Unless otherwise specified, all reagents used in this method shall be analytical
reagents; and the water shall be Class-I water in the laboratory specified in GB/T 6682.
4.1.2.1 Methanol (CH3OH): chromatographically pure.
4.1.2.2 Concentrated hydrochloric acid (HCl, density 1.19g/mL).
4.1.2.3 Trifluoroacetic acid: chromatographically pure.
4.1.2.4 Ammonium acetate.
4.1.2.5 Furosine: C12H17N2O4 • xHCl.
4.1.2.6 Hydrochloric acid solution (3mol/L): add 2.5 mL of concentrated hydrochloric
acid to 7.5mL of water; mix evenly.
4.1.2.7 Hydrochloric acid solution (10.6mol/L): add 88mL of concentrated hydrochloric
acid to 12mL of water, mix evenly.
4.1.2.8 Ammonium acetate solution (6g/L): accurately take 6g of ammonium acetate
to dissolve into water; make constant volume to 1L; pass through the 0.22µm aqueous
phase membrane; ultrasonically degas for 10min.
4.1.2.9 Ammonium acetate (6g/L) containing 0.1% trifluoroacetic acid solution:
accurately take 6g of ammonium acetate; dissolve into partial water; add 1mL of
trifluoroacetic acid; make constant volume to 1; pass through 0.22µm aqueous phase
membrane; ultrasonically degas for 10min.
4.1.2.10 Furosine standard stock solution (500.0mg/L): convert the furosine standard
substance as per the Net Peptide Content provided by the standard substance
certificate; then use 3mol/L hydrochloric acid solution to formulate into a standard stock
solution. It can be stored for 24 months at -20°C.
Example:
If the Net Peptide Content marked on the furosine standard substance certificate is 69.1%, then
take 7.24mg of furosine standard substance; use 3mol/L hydrochloric acid solution to dissolve
and make constant volume to 10mL; the concentration of standard stock solution is 500.0mg/L.
equivalent.
Column temperature: 35°C.
Mobile phase: 6g/L ammonium acetate containing 0.1% trifluoroacetic acid
aqueous solution is mobile phase A; methanol is mobile phase B; while pure
water is mobile phase C.
Elution conditions: mobile phase A. Isocratic elution, 0.4mL/min.
2) Determination
The mobile phase pure water and methanol should be used to wash the
chromatographic system; before the instrument is used, use mobile phase
pure water to transit; use mobile phase A to equilibrate the chromatographic
column at the flow rate of 0.4mL/min. Inject 0.5µL of 3mol/L hydrochloric acid
solution to check the purity of the solvent. Inject 0.5µL to-be-tested solution to
determine the furosien content. See Appendix A for chromatograms.
4.1.6 Result calculation
4.1.6.1 Furosine content in the specimen
The furosine is calculated by mass fraction F; the value of which is expressed in
mg/100g protein; and calculated as per Formula (1):
Where:
At – value of furosine peak area in the tested sample;
Astd – value of furosine peak area in the furosine standard solution;
Cstd – concentration of furosine standard solution, in mg/L;
D – when determining, the dilution factor (D=6);
m – protein concentration in the sample hydrolysate, in g/L,
The calculation result shall be retained to one digit after the decimal point.
4.1.6.2 Furosine content at the end of sterilization of pasteurized milk
At the end of sterilization of pasteurized milk, the furosine content is calculated by FT;
the value of which is expressed by mg/100g protein; and calculated as per Formula
(2):
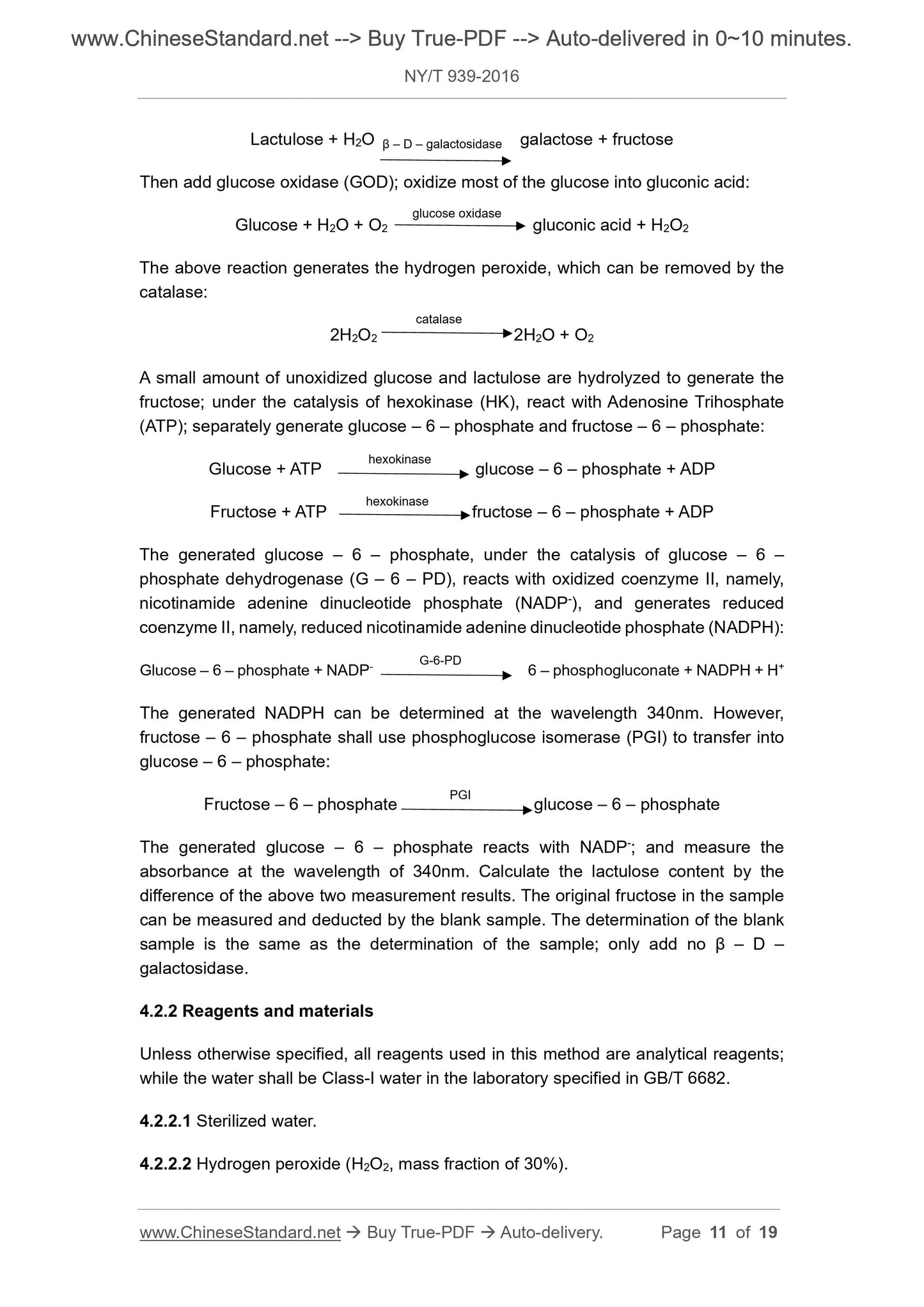
Lactulose + H2O galactose + fructose
Then add glucose oxidase (GOD); oxidize most of the glucose into gluconic acid:
Glucose + H2O + O2 gluconic acid + H2O2
The above reaction generates the hydrogen peroxide, which can be removed by the
catalase:
2H2O2 2H2O + O2
A small amount of unoxidized glucose and lactulose are hydrolyzed to generate the
fructose; under the catalysis of hexokinase (HK), react with Adenosine Trihosphate
(ATP); separately generate glucose – 6 – phosphate and fructose – 6 – phosphate:
Glucose + ATP glucose – 6 – phosphate + ADP
Fructose + ATP fructose – 6 – phosphate + ADP
The generated glucose – 6 – phosphate, under the catalysis of glucose – 6 –
phosphate dehydrogenase (G – 6 – PD), reacts with oxidized coenzyme II, namely,
nicotinamide adenine dinucleotide phosphate (NADP-), and generates reduced
coenzyme II, namely, reduced nicotinamide adenine dinucleotide phosphate (NADPH):
Glucose – 6 – phosphate + NADP- 6 – phosphogluconate + NADPH + H+
The generated NADPH can be determined at the wavelength 340nm. However,
fructose – 6 – phosphate shall use phosphoglucose isomerase (PGI) to transfer into
glucose – 6 – phosphate:
Fructose – 6 – phosphate glucose – 6 – phosphate
The generated glucose – 6 – phosphate reacts with NADP-; and measure the
absorbance at the wavelength of 340nm. Calculate the lactulose content by the
difference of the above two measurement results. The original fructose in the sample
can be measured and deducted by the blank sample. The determination of the blank
sample is the same as the determination of the sample; only add no β – D –
galactosidase.
4.2.2 Reagents and materials
Unless otherwise specified, all reagents used in this method are analytical reagents;
while the water shall be Class-I water in the laboratory specified in GB/T 6682.
4.2.2.1 Sterilized water.
4.2.2.2 Hydrogen peroxide (H2O2, mass fraction of 30%).
β – D – galactosidase
glucose oxidase
catalase
hexokinase
hexokinase
G-...
Get QUOTATION in 1-minute: Click NY/T 939-2016
Historical versions: NY/T 939-2016
Preview True-PDF (Reload/Scroll if blank)
NY/T 939-2016: Identification of reconstituted milk in pasteurized and UHT milk
NY/T 939-2016
AGRICULTURE INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 67.050
X 04
Replacing NY/T 939-2005
Identification of Reconstituted
Milk in Pasteurized and UHT Milk
ISSUED ON: MARCH 23, 2016
IMPLEMENTED ON: APRIL 01, 2016
Issued by: Ministry of Agriculture of PRC
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative References ... 4
3 Terms and Definitions ... 4
4 Test Methods ... 5
5 Identification of Reconstituted Milk ... 16
Appendix A (Informative) Furosine Liquid Chromatogram ... 18
Identification of Reconstituted
Milk in Pasteurized and UHT Milk
1 Scope
This Standard specifies the identification method of reconstituted milk in pasteurized
and UHT milk.
This Standard is applicable to the pasteurized and UHT milk.
2 Normative References
The following documents are essential to the application of this document. For the
dated documents, only the versions with the dates indicated are applicable to this
document; for the undated documents, only the latest version (including all the
amendments) are applicable to this document.
GB 5009.5 Determination of Protein in Foods
GB/T 6682 Water for Laboratory Use – Specifications
GB/T 10111 Generation of Random Numbers and Procedures Applied to Sampling
Inspection for Product Quality
3 Terms and Definitions
For the purposes of this document, the following terms and definitions apply.
3.1 Raw milk
The normal milk extruded from the breasts of the healthy dairy animals, it meets the
relevant national requirements and has no ingredient changes.
3.2 Reconstituted milk
The milk obtained by mixing a dried or concentrated dairy product with water in
proportion.
3.3 Heat treatment
4.1.1 Principle
Hydrolyze the specimen by hydrochloric acid, then determine its protein content; after
diluting, the hydrolysate shall be analyzed by high performance liquid chromatography
(HPLC) or ultra-high-performance liquid chromatography (UPLC) under ultraviolet
(wavelength 280nm) detector and quantify by external standard method.
4.1.2 Reagents and materials
Unless otherwise specified, all reagents used in this method shall be analytical
reagents; and the water shall be Class-I water in the laboratory specified in GB/T 6682.
4.1.2.1 Methanol (CH3OH): chromatographically pure.
4.1.2.2 Concentrated hydrochloric acid (HCl, density 1.19g/mL).
4.1.2.3 Trifluoroacetic acid: chromatographically pure.
4.1.2.4 Ammonium acetate.
4.1.2.5 Furosine: C12H17N2O4 • xHCl.
4.1.2.6 Hydrochloric acid solution (3mol/L): add 2.5 mL of concentrated hydrochloric
acid to 7.5mL of water; mix evenly.
4.1.2.7 Hydrochloric acid solution (10.6mol/L): add 88mL of concentrated hydrochloric
acid to 12mL of water, mix evenly.
4.1.2.8 Ammonium acetate solution (6g/L): accurately take 6g of ammonium acetate
to dissolve into water; make constant volume to 1L; pass through the 0.22µm aqueous
phase membrane; ultrasonically degas for 10min.
4.1.2.9 Ammonium acetate (6g/L) containing 0.1% trifluoroacetic acid solution:
accurately take 6g of ammonium acetate; dissolve into partial water; add 1mL of
trifluoroacetic acid; make constant volume to 1; pass through 0.22µm aqueous phase
membrane; ultrasonically degas for 10min.
4.1.2.10 Furosine standard stock solution (500.0mg/L): convert the furosine standard
substance as per the Net Peptide Content provided by the standard substance
certificate; then use 3mol/L hydrochloric acid solution to formulate into a standard stock
solution. It can be stored for 24 months at -20°C.
Example:
If the Net Peptide Content marked on the furosine standard substance certificate is 69.1%, then
take 7.24mg of furosine standard substance; use 3mol/L hydrochloric acid solution to dissolve
and make constant volume to 10mL; the concentration of standard stock solution is 500.0mg/L.
equivalent.
Column temperature: 35°C.
Mobile phase: 6g/L ammonium acetate containing 0.1% trifluoroacetic acid
aqueous solution is mobile phase A; methanol is mobile phase B; while pure
water is mobile phase C.
Elution conditions: mobile phase A. Isocratic elution, 0.4mL/min.
2) Determination
The mobile phase pure water and methanol should be used to wash the
chromatographic system; before the instrument is used, use mobile phase
pure water to transit; use mobile phase A to equilibrate the chromatographic
column at the flow rate of 0.4mL/min. Inject 0.5µL of 3mol/L hydrochloric acid
solution to check the purity of the solvent. Inject 0.5µL to-be-tested solution to
determine the furosien content. See Appendix A for chromatograms.
4.1.6 Result calculation
4.1.6.1 Furosine content in the specimen
The furosine is calculated by mass fraction F; the value of which is expressed in
mg/100g protein; and calculated as per Formula (1):
Where:
At – value of furosine peak area in the tested sample;
Astd – value of furosine peak area in the furosine standard solution;
Cstd – concentration of furosine standard solution, in mg/L;
D – when determining, the dilution factor (D=6);
m – protein concentration in the sample hydrolysate, in g/L,
The calculation result shall be retained to one digit after the decimal point.
4.1.6.2 Furosine content at the end of sterilization of pasteurized milk
At the end of sterilization of pasteurized milk, the furosine content is calculated by FT;
the value of which is expressed by mg/100g protein; and calculated as per Formula
(2):
Lactulose + H2O galactose + fructose
Then add glucose oxidase (GOD); oxidize most of the glucose into gluconic acid:
Glucose + H2O + O2 gluconic acid + H2O2
The above reaction generates the hydrogen peroxide, which can be removed by the
catalase:
2H2O2 2H2O + O2
A small amount of unoxidized glucose and lactulose are hydrolyzed to generate the
fructose; under the catalysis of hexokinase (HK), react with Adenosine Trihosphate
(ATP); separately generate glucose – 6 – phosphate and fructose – 6 – phosphate:
Glucose + ATP glucose – 6 – phosphate + ADP
Fructose + ATP fructose – 6 – phosphate + ADP
The generated glucose – 6 – phosphate, under the catalysis of glucose – 6 –
phosphate dehydrogenase (G – 6 – PD), reacts with oxidized coenzyme II, namely,
nicotinamide adenine dinucleotide phosphate (NADP-), and generates reduced
coenzyme II, namely, reduced nicotinamide adenine dinucleotide phosphate (NADPH):
Glucose – 6 – phosphate + NADP- 6 – phosphogluconate + NADPH + H+
The generated NADPH can be determined at the wavelength 340nm. However,
fructose – 6 – phosphate shall use phosphoglucose isomerase (PGI) to transfer into
glucose – 6 – phosphate:
Fructose – 6 – phosphate glucose – 6 – phosphate
The generated glucose – 6 – phosphate reacts with NADP-; and measure the
absorbance at the wavelength of 340nm. Calculate the lactulose content by the
difference of the above two measurement results. The original fructose in the sample
can be measured and deducted by the blank sample. The determination of the blank
sample is the same as the determination of the sample; only add no β – D –
galactosidase.
4.2.2 Reagents and materials
Unless otherwise specified, all reagents used in this method are analytical reagents;
while the water shall be Class-I water in the laboratory specified in GB/T 6682.
4.2.2.1 Sterilized water.
4.2.2.2 Hydrogen peroxide (H2O2, mass fraction of 30%).
β – D – galactosidase
glucose oxidase
catalase
hexokinase
hexokinase
G-...
Share