1
/
of
9
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1704.1-2020 English PDF (YYT1704.1-2020)
YY/T 1704.1-2020 English PDF (YYT1704.1-2020)
Regular price
$125.00 USD
Regular price
Sale price
$125.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY/T 1704.1-2020
Historical versions: YY/T 1704.1-2020
Preview True-PDF (Reload/Scroll if blank)
YY/T 1704.1-2020: Cervical dilator for single use--Part 1: Gradual dilator
YY/T 1704.1-2020
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.30
C 36
Cervical dilator for single use - Part 1: Gradual dilator
ISSUED ON: FEBRUARY 21, 2020
IMPLEMENTED ON: JANUARY 01, 2021
Issued by: National Medical Products Administration
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative references ... 4
3 Classification ... 4
4 Requirements ... 6
5 Test methods ... 7
6 Marks, packaging and instruction manual ... 9
7 Transport, storage ... 9
Cervical dilator for single use - Part 1: Gradual dilator
1 Scope
This Part of YY/T 1704 specifies the classification, requirements, test methods,
marks, packaging and instruction manual, transport, storage and sterilization
period for gradual cervical dilator for single use (hereinafter referred to as the
dilator).
This Part is applicable to cervical dilator for single use. This product is used for
dilatation of the cervix in obstetrics and gynecology and family planning
departments.
This Part is not applicable to the cervical dilator made of metal.
2 Normative references
The following referenced documents are indispensable for the application of
this document. For dated references, only the edition cited applies. For undated
references, the latest edition of the referenced document (including any
amendments) applies.
GB/T 14233.1-2008, Test methods for infusion transfusion injection
equipment for medical use - Part 1: Chemical analysis methods
GB/T 16886.1, Biological evaluation of medical devices - Part 1: Evaluation
and testing
YY/T 0171, Surgical instruments - Packaging, Marking and Instructions
Pharmacopoeia of the People's Republic of China (2015 Edition) Part IV
3 Classification
3.1 Type
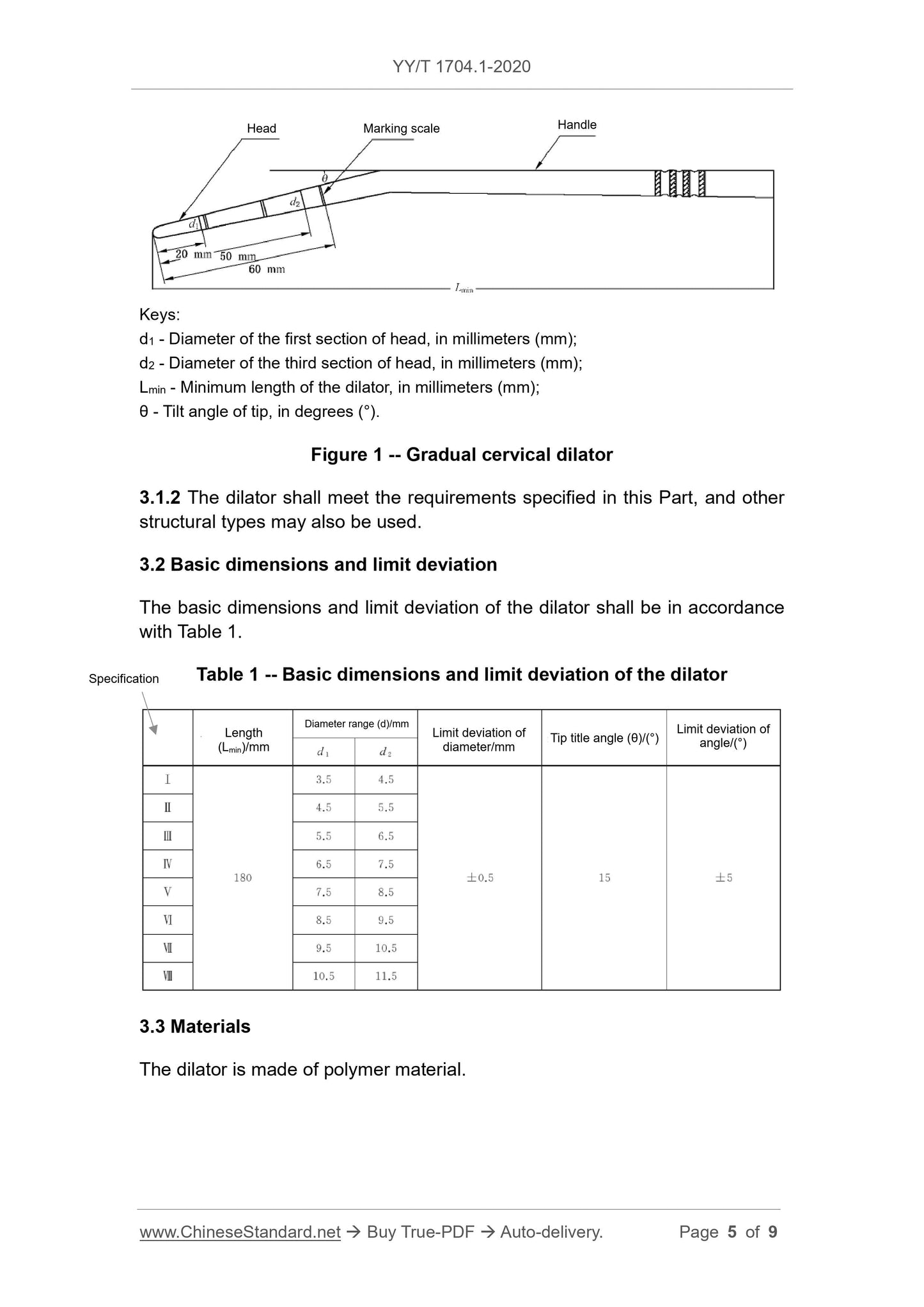
3.1.1 The dilator is the expansion section within 60mm of the ring line from the
head end. It is usually composed of 3 gradual units. Each unit is about 20mm
long.
4 Requirements
4.1 Appearance
4.1.1 The head of the dilator shall be round and smooth, and no fibers shall be
pulled out.
4.1.2 The marks of the dilator shall be clear and obvious.
4.1.3 The overall dilator shall be smooth and uniform in color, and there shall
be no cracks, burrs, plastic flow, or defects.
4.2 Dimensions
The gradual diameter range d1 and d2 of the dilator, the tilt angle θ of the tip
shall meet the requirements of Table 1.
4.3 Physical properties
4.3.1 The dilator shall be able to withstand 50N axial tension without breaking.
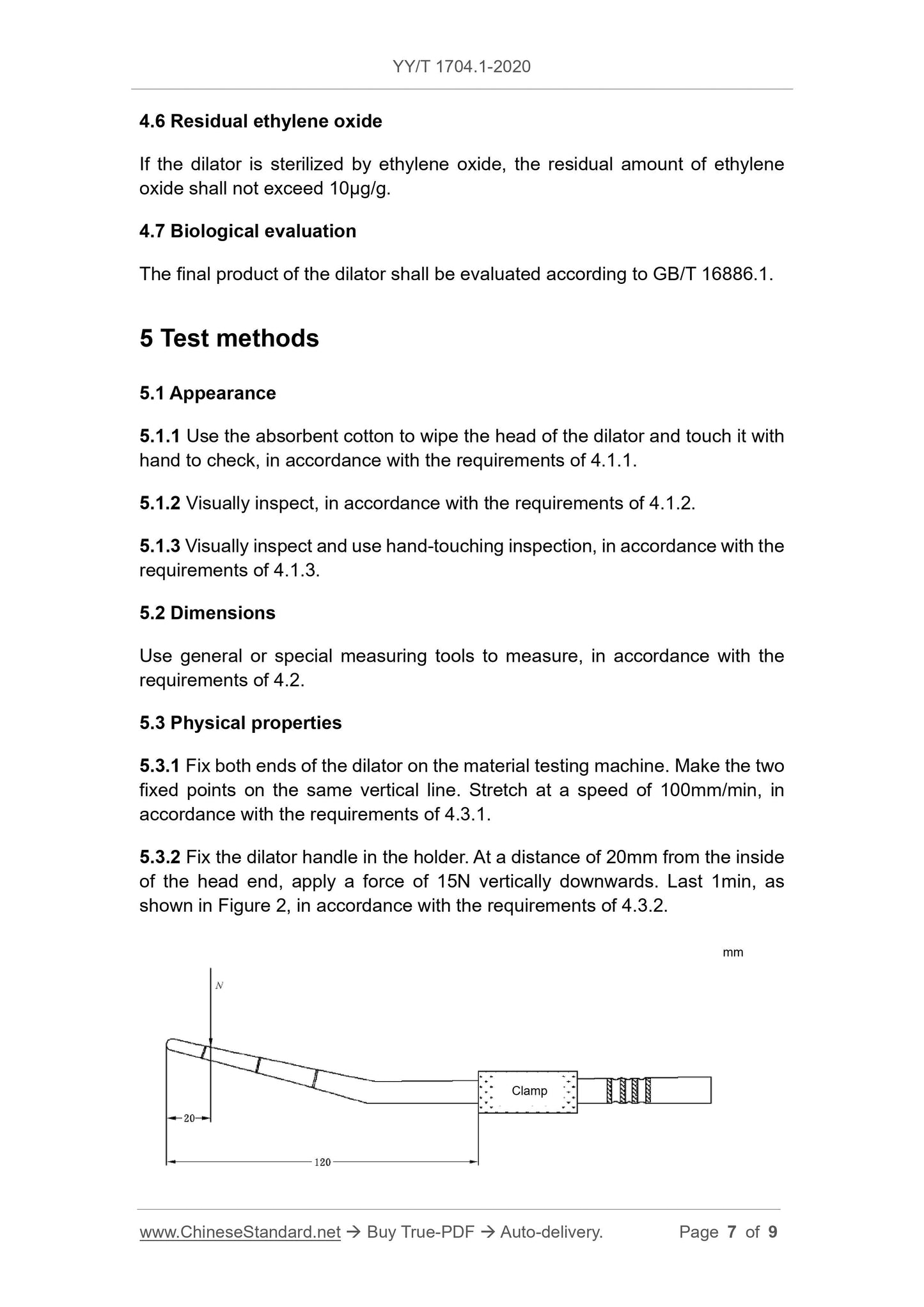
4.3.2 Apply a force of 15N to the head of the dilator, and there shall be no
obvious deformation or fracture.
NOTE: Obvious deformation can be understood as the tip tilt angle θ≤0° after applying
force.
4.4 Chemical properties
4.4.1 Heavy metals
The total content of heavy metals in the dilator test solution shall not exceed
1μg/mL.
4.4.2 PH
Compare the dilator test solution with the blank solution. The difference in pH
shall not exceed 1.5.
4.4.3 UV absorbance
The absorbance of the dilator test solution in the wavelength range of
250nm~320nm shall not exceed 0.1.
4.5 Sterilization
The dilator shall be sterilized by a confirmed sterilization process, and the
sterilized dilator shall be sterile.
NOTE: N is the loading force.
Figure 2 -- Schematic diagram of bending performance test of the dilator
5.4 Chemical properties
5.4.1 Test solution preparation
Take sample. Add water at a ratio: 0.2g of sample to 1mL of water. Keep
constant temperature at (37±1)°C for 8h. Separate the sample from water. Cool
to room temperature as the test solution. Take the same volume of water and
place it in a glass container. Prepare blank control solution in the same way.
5.4.2 Heavy metals
Test according to the method specified in 5.6.1 of GB/T 14233.1-2008, in
accordance with the requirements of 4.4.1.
5.4.3 PH
Test according to the method specified in 5.4.1 of GB/T 14233.1-2008, in
accordance with the requirements of 4.4.2.
5.4.4 UV absorbance
Test according to the method specified in 5.7 of GB/T 14233.1-2008, in
accordance with the requirements of 4.4.3.
5.5 Sterilization
The inspection is carried out in accordance with the method stipulated in the
fourth part 1101 sterile inspection method of the "Pharmacopoeia of the
People's Republic of China" (Edition 2015), in accordance with the
requirements of 4.5.
NOTE: Each sterilization batch undergoes an effective monitoring process to make the
product sterile.
5.6 Residual ethylene oxide
Test according to the method in Clause 9 of GB/T 14233.1-2008, in accordance
with the requirements of 4.6.
5.7 Biological evaluation
Evaluate according to the corresponding method of biological evaluation, in
accordance with the requirements of 4.7.
Get QUOTATION in 1-minute: Click YY/T 1704.1-2020
Historical versions: YY/T 1704.1-2020
Preview True-PDF (Reload/Scroll if blank)
YY/T 1704.1-2020: Cervical dilator for single use--Part 1: Gradual dilator
YY/T 1704.1-2020
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.30
C 36
Cervical dilator for single use - Part 1: Gradual dilator
ISSUED ON: FEBRUARY 21, 2020
IMPLEMENTED ON: JANUARY 01, 2021
Issued by: National Medical Products Administration
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative references ... 4
3 Classification ... 4
4 Requirements ... 6
5 Test methods ... 7
6 Marks, packaging and instruction manual ... 9
7 Transport, storage ... 9
Cervical dilator for single use - Part 1: Gradual dilator
1 Scope
This Part of YY/T 1704 specifies the classification, requirements, test methods,
marks, packaging and instruction manual, transport, storage and sterilization
period for gradual cervical dilator for single use (hereinafter referred to as the
dilator).
This Part is applicable to cervical dilator for single use. This product is used for
dilatation of the cervix in obstetrics and gynecology and family planning
departments.
This Part is not applicable to the cervical dilator made of metal.
2 Normative references
The following referenced documents are indispensable for the application of
this document. For dated references, only the edition cited applies. For undated
references, the latest edition of the referenced document (including any
amendments) applies.
GB/T 14233.1-2008, Test methods for infusion transfusion injection
equipment for medical use - Part 1: Chemical analysis methods
GB/T 16886.1, Biological evaluation of medical devices - Part 1: Evaluation
and testing
YY/T 0171, Surgical instruments - Packaging, Marking and Instructions
Pharmacopoeia of the People's Republic of China (2015 Edition) Part IV
3 Classification
3.1 Type
3.1.1 The dilator is the expansion section within 60mm of the ring line from the
head end. It is usually composed of 3 gradual units. Each unit is about 20mm
long.
4 Requirements
4.1 Appearance
4.1.1 The head of the dilator shall be round and smooth, and no fibers shall be
pulled out.
4.1.2 The marks of the dilator shall be clear and obvious.
4.1.3 The overall dilator shall be smooth and uniform in color, and there shall
be no cracks, burrs, plastic flow, or defects.
4.2 Dimensions
The gradual diameter range d1 and d2 of the dilator, the tilt angle θ of the tip
shall meet the requirements of Table 1.
4.3 Physical properties
4.3.1 The dilator shall be able to withstand 50N axial tension without breaking.
4.3.2 Apply a force of 15N to the head of the dilator, and there shall be no
obvious deformation or fracture.
NOTE: Obvious deformation can be understood as the tip tilt angle θ≤0° after applying
force.
4.4 Chemical properties
4.4.1 Heavy metals
The total content of heavy metals in the dilator test solution shall not exceed
1μg/mL.
4.4.2 PH
Compare the dilator test solution with the blank solution. The difference in pH
shall not exceed 1.5.
4.4.3 UV absorbance
The absorbance of the dilator test solution in the wavelength range of
250nm~320nm shall not exceed 0.1.
4.5 Sterilization
The dilator shall be sterilized by a confirmed sterilization process, and the
sterilized dilator shall be sterile.
NOTE: N is the loading force.
Figure 2 -- Schematic diagram of bending performance test of the dilator
5.4 Chemical properties
5.4.1 Test solution preparation
Take sample. Add water at a ratio: 0.2g of sample to 1mL of water. Keep
constant temperature at (37±1)°C for 8h. Separate the sample from water. Cool
to room temperature as the test solution. Take the same volume of water and
place it in a glass container. Prepare blank control solution in the same way.
5.4.2 Heavy metals
Test according to the method specified in 5.6.1 of GB/T 14233.1-2008, in
accordance with the requirements of 4.4.1.
5.4.3 PH
Test according to the method specified in 5.4.1 of GB/T 14233.1-2008, in
accordance with the requirements of 4.4.2.
5.4.4 UV absorbance
Test according to the method specified in 5.7 of GB/T 14233.1-2008, in
accordance with the requirements of 4.4.3.
5.5 Sterilization
The inspection is carried out in accordance with the method stipulated in the
fourth part 1101 sterile inspection method of the "Pharmacopoeia of the
People's Republic of China" (Edition 2015), in accordance with the
requirements of 4.5.
NOTE: Each sterilization batch undergoes an effective monitoring process to make the
product sterile.
5.6 Residual ethylene oxide
Test according to the method in Clause 9 of GB/T 14233.1-2008, in accordance
with the requirements of 4.6.
5.7 Biological evaluation
Evaluate according to the corresponding method of biological evaluation, in
accordance with the requirements of 4.7.
Share