1
/
of
9
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 1704.1-2020 English PDF (YY/T1704.1-2020)
YY/T 1704.1-2020 English PDF (YY/T1704.1-2020)
Regular price
$125.00 USD
Regular price
Sale price
$125.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY/T 1704.1-2020 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1704.1-2020
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1704.1-2020: Cervical dilator for single use--Part 1: Gradual dilator
YY/T 1704.1-2020
Cervical dilator for single use--Part 1.Gradual dilator
ICS 11.040.30
C36
People's Republic of China Pharmaceutical Industry Standard
One-time use cervical dilator Part 1.Progressive
2020-02-21 released
2021-01-01 implementation
Issued by the State Drug Administration
Preface
YY/T 1704 "Single Use Cervical Dilator" is divided into the following 3 parts.
---Part 1.Progressive;
---Part 2.Expansion;
---Part 3.Balloon type.
This part is Part 1 of YY/T 1704.
This section was drafted in accordance with the rules given in GB/T 1.1-2009.
Please note that certain contents of this document may involve patents. The issuing agency of this document is not responsible for identifying these patents.
This part was proposed by the State Drug Administration.
This part is under the jurisdiction of the National Family Planning Device Standardization Technical Committee (SAC/TC169).
Drafting organizations of this section. Shanghai Jiabao Medical Healthcare Technology Co., Ltd., Shanghai Heng Instrument Factory Co., Ltd., Shanghai Medical Equipment Inspection
Test.
The main drafters of this section. Xu Ming, Wang Huyu, Weng Binghao, Wu Zhimin, Yao Tianping, Wu Yaojin, Zou Bing.
One-time use cervical dilator Part 1.Progressive
1 Scope
This part of YY/T 1704 specifies the classification, requirements and tests of progressive, single-use cervical dilators (hereinafter referred to as dilators)
Methods, signs, packaging and instructions for use, transportation, storage and sterilization period of validity.
This section applies to a single-use cervical dilator, the product for obstetrics and gynecology, family planning departments to dilate the cervix.
This section does not apply to cervical dilators made of metal.
2 Normative references
The following documents are indispensable for the application of this document. For dated reference documents, only the dated version applies to this article
Pieces. For undated references, the latest version (including all amendments) applies to this document.
GB/T 14233.1-2008 Medical infusion, blood transfusion, and injection equipment inspection methods Part 1.Chemical analysis methods
GB/T 16886.1 Biological Evaluation of Medical Devices Part 1.Evaluation and Testing in the Process of Risk Management
YY/T 0171 Surgical instrument packaging, marking and instruction manual
Pharmacopoeia of the People's Republic of China (2015 Edition) Part IV
3 categories
3.1 Type
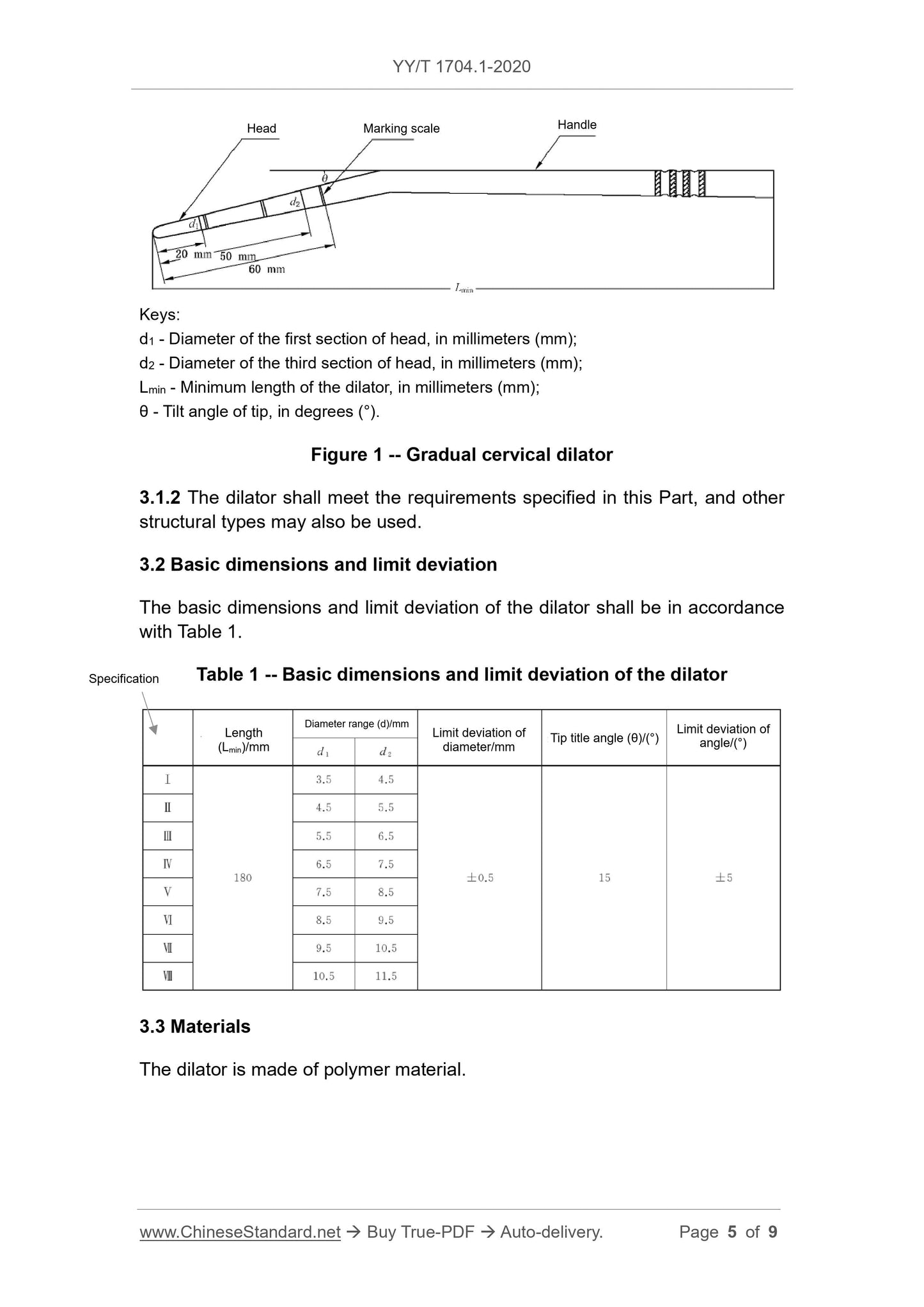
3.1.1 The expander is the expansion section within 60mm of the ring line from the head end, usually composed of 3 progressive units, each unit is about 20mm long.
Figure 1 Progressive cervical dilator
3.1.2 The expander should meet the requirements specified in this section, and other structural types may also be used.
3.2 Basic dimensions and limit deviations
The basic size and limit deviation of the expander shall be in accordance with the provisions of Table 1.
3.3 Materials
The expander is made of polymer material.
4 requirements
4.1 Appearance
4.1.1 The head of the dilator should be round and smooth, and no fibers should be pulled out.
4.1.2 The marking of the expander shall be clear and obvious.
4.1.3 The entire expander should be smooth and uniform in color, and there should be no cracks, burrs, plastic flow, or defects.
4.2 Dimensions
The progressive diameter range d1 and d2 of the dilator, and the tilt angle θ of the head end should meet the requirements of Table 1.
4.3 Physical properties
4.3.1 The expander should be able to withstand 50N axial tension without breaking.
4.3.2 Applying a force of 15N to the head of the expander should not cause obvious deformation or fracture.
Note. Obvious deformation can be understood as the tip tilt angle θ≤0° after applying force.
4.4 Chemical properties
4.4.1 Heavy metals
The total content of heavy metals in the dilator test solution should not exceed 1μg/mL.
4.4.2 pH
Comparing the dilator test solution with the blank solution, the difference in pH should not exceed 1.5.
4.4.3 UV absorbance
The absorbance of the dilator test solution in the wavelength range of 250nm~320nm should not exceed 0.1.
4.5 Sterility
The dilator should be sterilized by a confirmed sterilization process, and the dilator after sterilization should be sterile.
4.6 Residual ethylene oxide
If the expander is sterilized with ethylene oxide, the residual amount of ethylene oxide should not exceed 10μg/g.
4.7 Biological evaluation
The final product of the expander should be evaluated according to GB/T 16886.1.
5 Test method
5.1 Appearance
5.1.1 Wipe the head of the dilator with absorbent cotton and touch it with your hand to check that it should meet the requirements of 4.1.1.
5.1.2 Visual observation should meet the requirements of 4.1.2.
5.1.3 Use visual observation and hand touching for inspection, which should meet the requirements of 4.1.3.
5.2 Dimensions
Use general-purpose or special-purpose measuring tools to measure, and it should comply with 4.2.
5.3 Physical properties
5.3.1 Fix both ends of the expander on the material testing machine so that the two fixed points are on the same vertical line, and proceed at a speed of 100mm/min.
Stretching should meet the requirements of 4.3.1.
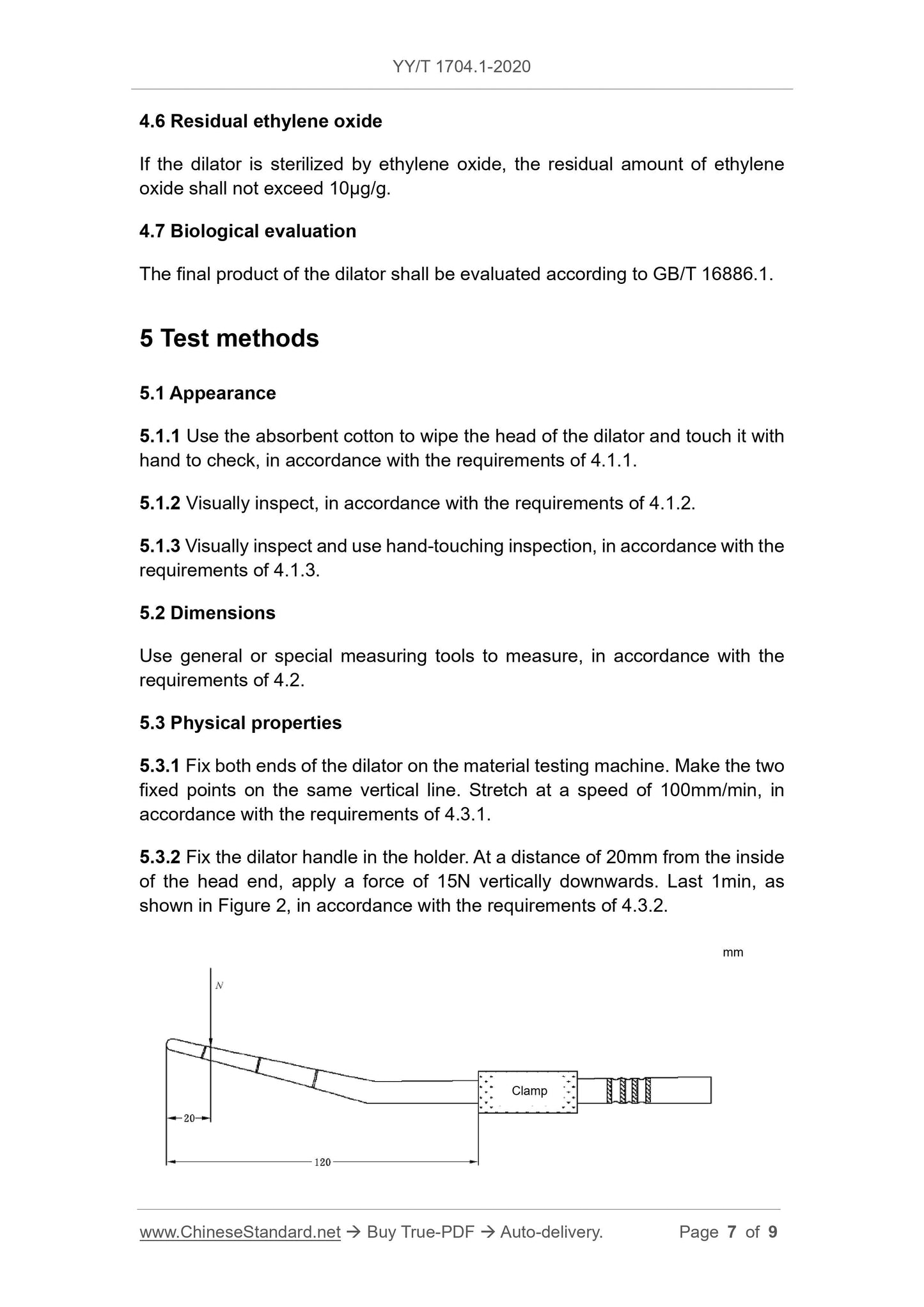
5.3.2 Fix the handle of the expander in the holder, and apply a force of 15N vertically downward at a distance of 20mm from the inner side of the head end for 1 min.
as shown in picture 2.Should meet the requirements of 4.3.2.
Figure 2 Schematic diagram of the bending performance test of the expander
5.4 Chemical properties
5.4.1 Preparation of test solution
Take the sample, add water according to the ratio of 0.2g sample to 1mL water, keep the temperature at (37±1)℃ for 8h, separate the sample from the water, and cool to the room
Warm as the test solution. Take the same volume of water and place it in a glass container, and prepare a blank control solution in the same way.
5.4.2 Heavy metals
The test is carried out according to the method specified in 5.6.1 of GB/T 14233.1-2008, and it shall meet the requirements of 4.4.1.
5.4.3 pH
The test is carried out according to the method specified in 5.4.1 of GB/T 14233.1-2008, which shall meet the requirements of 4.4.2.
5.4.4 UV absorbance
Test according to the method in GB/T 14233.1-2008 5.7, and it should meet the requirements of 4.4.3.
5.5 Sterility
The inspection is carried out in accordance with the method stipulated in the fourth part 1101 of the "Pharmacopoeia of the People's Republic of China" (2015 edition), and should meet 4.5
Provisions.
Note. Each sterilization batch undergoes an effective monitoring process to make the product sterile.
5.6 Residual ethylene oxide
According to the method in Chapter 9 of GB/T 14233.1-2008, the test should meet the requirements of 4.6.
5.7 Biological evaluation
According to the corresponding method of biological evaluation, it should meet the requirements of 4.7.
6 Marks, packaging and instructions for use
The expander logo, packaging and instructions for use should comply with YY/T 0171.
7 Transportation and storage
7.1 Transportation
Packing and transportation requirements are in accordance with the provisions of the order contract.
7.2 Storage
7.2.1 The words and marks on the packing box should be clear and not obscure due to a long time.
7.2.2 The packaged dilator should be stored in a room with a relative humidity of no more than 80%, no corrosive gas, and good ventilation.
8 Sterilization validity period
The expander sterilized after the packaging bag is sealed should be marked with the sterilization loss calculated from the date of sterilization under the conditions of observing the storage rules.
Expiration date.
Get Quotation: Click YY/T 1704.1-2020 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 1704.1-2020
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 1704.1-2020: Cervical dilator for single use--Part 1: Gradual dilator
YY/T 1704.1-2020
Cervical dilator for single use--Part 1.Gradual dilator
ICS 11.040.30
C36
People's Republic of China Pharmaceutical Industry Standard
One-time use cervical dilator Part 1.Progressive
2020-02-21 released
2021-01-01 implementation
Issued by the State Drug Administration
Preface
YY/T 1704 "Single Use Cervical Dilator" is divided into the following 3 parts.
---Part 1.Progressive;
---Part 2.Expansion;
---Part 3.Balloon type.
This part is Part 1 of YY/T 1704.
This section was drafted in accordance with the rules given in GB/T 1.1-2009.
Please note that certain contents of this document may involve patents. The issuing agency of this document is not responsible for identifying these patents.
This part was proposed by the State Drug Administration.
This part is under the jurisdiction of the National Family Planning Device Standardization Technical Committee (SAC/TC169).
Drafting organizations of this section. Shanghai Jiabao Medical Healthcare Technology Co., Ltd., Shanghai Heng Instrument Factory Co., Ltd., Shanghai Medical Equipment Inspection
Test.
The main drafters of this section. Xu Ming, Wang Huyu, Weng Binghao, Wu Zhimin, Yao Tianping, Wu Yaojin, Zou Bing.
One-time use cervical dilator Part 1.Progressive
1 Scope
This part of YY/T 1704 specifies the classification, requirements and tests of progressive, single-use cervical dilators (hereinafter referred to as dilators)
Methods, signs, packaging and instructions for use, transportation, storage and sterilization period of validity.
This section applies to a single-use cervical dilator, the product for obstetrics and gynecology, family planning departments to dilate the cervix.
This section does not apply to cervical dilators made of metal.
2 Normative references
The following documents are indispensable for the application of this document. For dated reference documents, only the dated version applies to this article
Pieces. For undated references, the latest version (including all amendments) applies to this document.
GB/T 14233.1-2008 Medical infusion, blood transfusion, and injection equipment inspection methods Part 1.Chemical analysis methods
GB/T 16886.1 Biological Evaluation of Medical Devices Part 1.Evaluation and Testing in the Process of Risk Management
YY/T 0171 Surgical instrument packaging, marking and instruction manual
Pharmacopoeia of the People's Republic of China (2015 Edition) Part IV
3 categories
3.1 Type
3.1.1 The expander is the expansion section within 60mm of the ring line from the head end, usually composed of 3 progressive units, each unit is about 20mm long.
Figure 1 Progressive cervical dilator
3.1.2 The expander should meet the requirements specified in this section, and other structural types may also be used.
3.2 Basic dimensions and limit deviations
The basic size and limit deviation of the expander shall be in accordance with the provisions of Table 1.
3.3 Materials
The expander is made of polymer material.
4 requirements
4.1 Appearance
4.1.1 The head of the dilator should be round and smooth, and no fibers should be pulled out.
4.1.2 The marking of the expander shall be clear and obvious.
4.1.3 The entire expander should be smooth and uniform in color, and there should be no cracks, burrs, plastic flow, or defects.
4.2 Dimensions
The progressive diameter range d1 and d2 of the dilator, and the tilt angle θ of the head end should meet the requirements of Table 1.
4.3 Physical properties
4.3.1 The expander should be able to withstand 50N axial tension without breaking.
4.3.2 Applying a force of 15N to the head of the expander should not cause obvious deformation or fracture.
Note. Obvious deformation can be understood as the tip tilt angle θ≤0° after applying force.
4.4 Chemical properties
4.4.1 Heavy metals
The total content of heavy metals in the dilator test solution should not exceed 1μg/mL.
4.4.2 pH
Comparing the dilator test solution with the blank solution, the difference in pH should not exceed 1.5.
4.4.3 UV absorbance
The absorbance of the dilator test solution in the wavelength range of 250nm~320nm should not exceed 0.1.
4.5 Sterility
The dilator should be sterilized by a confirmed sterilization process, and the dilator after sterilization should be sterile.
4.6 Residual ethylene oxide
If the expander is sterilized with ethylene oxide, the residual amount of ethylene oxide should not exceed 10μg/g.
4.7 Biological evaluation
The final product of the expander should be evaluated according to GB/T 16886.1.
5 Test method
5.1 Appearance
5.1.1 Wipe the head of the dilator with absorbent cotton and touch it with your hand to check that it should meet the requirements of 4.1.1.
5.1.2 Visual observation should meet the requirements of 4.1.2.
5.1.3 Use visual observation and hand touching for inspection, which should meet the requirements of 4.1.3.
5.2 Dimensions
Use general-purpose or special-purpose measuring tools to measure, and it should comply with 4.2.
5.3 Physical properties
5.3.1 Fix both ends of the expander on the material testing machine so that the two fixed points are on the same vertical line, and proceed at a speed of 100mm/min.
Stretching should meet the requirements of 4.3.1.
5.3.2 Fix the handle of the expander in the holder, and apply a force of 15N vertically downward at a distance of 20mm from the inner side of the head end for 1 min.
as shown in picture 2.Should meet the requirements of 4.3.2.
Figure 2 Schematic diagram of the bending performance test of the expander
5.4 Chemical properties
5.4.1 Preparation of test solution
Take the sample, add water according to the ratio of 0.2g sample to 1mL water, keep the temperature at (37±1)℃ for 8h, separate the sample from the water, and cool to the room
Warm as the test solution. Take the same volume of water and place it in a glass container, and prepare a blank control solution in the same way.
5.4.2 Heavy metals
The test is carried out according to the method specified in 5.6.1 of GB/T 14233.1-2008, and it shall meet the requirements of 4.4.1.
5.4.3 pH
The test is carried out according to the method specified in 5.4.1 of GB/T 14233.1-2008, which shall meet the requirements of 4.4.2.
5.4.4 UV absorbance
Test according to the method in GB/T 14233.1-2008 5.7, and it should meet the requirements of 4.4.3.
5.5 Sterility
The inspection is carried out in accordance with the method stipulated in the fourth part 1101 of the "Pharmacopoeia of the People's Republic of China" (2015 edition), and should meet 4.5
Provisions.
Note. Each sterilization batch undergoes an effective monitoring process to make the product sterile.
5.6 Residual ethylene oxide
According to the method in Chapter 9 of GB/T 14233.1-2008, the test should meet the requirements of 4.6.
5.7 Biological evaluation
According to the corresponding method of biological evaluation, it should meet the requirements of 4.7.
6 Marks, packaging and instructions for use
The expander logo, packaging and instructions for use should comply with YY/T 0171.
7 Transportation and storage
7.1 Transportation
Packing and transportation requirements are in accordance with the provisions of the order contract.
7.2 Storage
7.2.1 The words and marks on the packing box should be clear and not obscure due to a long time.
7.2.2 The packaged dilator should be stored in a room with a relative humidity of no more than 80%, no corrosive gas, and good ventilation.
8 Sterilization validity period
The expander sterilized after the packaging bag is sealed should be marked with the sterilization loss calculated from the date of sterilization under the conditions of observing the storage rules.
Expiration date.
Share