1
/
of
6
www.ChineseStandard.us -- Field Test Asia Pte. Ltd.
GB 1903.50-2020 English PDF
GB 1903.50-2020 English PDF
Regular price
$170.00
Regular price
Sale price
$170.00
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
GB 1903.50-2020: National food safety standard - Food nutritional fortification substance - Cholecalciferol (Vitamin D3)
Delivery: 9 seconds. Download (& Email) true-PDF + Invoice.
Get Quotation: Click GB 1903.50-2020 (Self-service in 1-minute)
Historical versions (Master-website): GB 1903.50-2020
Preview True-PDF (Reload/Scroll-down if blank)
GB 1903.50-2020
GB
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National food safety standard - Food nutritional
fortification substance - Cholecalciferol (Vitamin D3)
食品营养强化剂 胆钙化醇(维生素 D3)
ISSUED ON: SEPTEMBER 11, 2020
IMPLEMENTED ON: MARCH 11, 2021
Issued by: National Health Commission of the People's Republic of China;
State Administration for Market Regulation.
Table of Contents
1 Scope ... 3
2 Chemical name, structural formula, molecular formula, relative molecular
mass ... 3
3 Technical requirements ... 4
Annex A Inspection methods ... 5
Annex B Standard infrared spectrum of vitamin D3 ... 12
Annex C HPLC reference chromatogram of vitamin D3 and related substances
... 13
National food safety standard - Food nutritional
fortification substance - Cholecalciferol (Vitamin D3)
1 Scope
This Standard is applicable to food nutritional fortification substance -
cholecalciferol (Vitamin D3), which uses lanolin cholesterol as raw material, by
chemical synthesis, to obtain 7-dehydrocholesterol, then is made through UV
irradiation, refining and other processes.
2 Chemical name, structural formula, molecular
formula, relative molecular mass
2.1 Chemical name
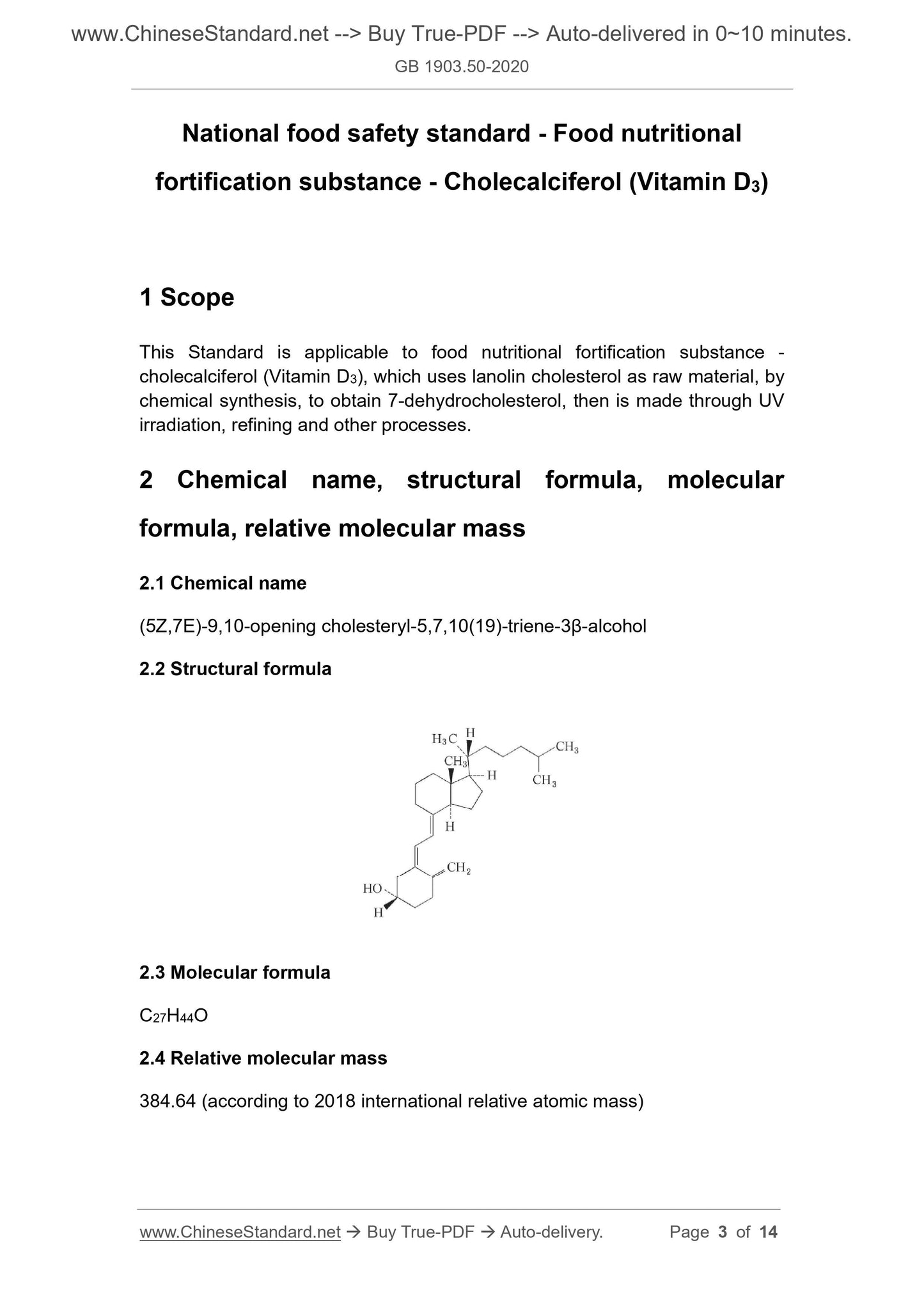
(5Z,7E)-9,10-opening cholesteryl-5,7,10(19)-triene-3β-alcohol
2.2 Structural formula
2.3 Molecular formula
C27H44O
2.4 Relative molecular mass
384.64 (according to 2018 international relative atomic mass)
Annex A
Inspection methods
A.1 General provisions
All reagents and water used in this Standard, when other requirements are not
specified, refer to analytically-pure reagents or of above specifications and
grade 3 water specified in GB/T 6682. Standard solutions used in the test,
standard solutions for impurity determination, preparations and products, when
other requirements are not specified, are prepared in accordance with GB/T
601, GB/T 602 and GB/T 603. Solution used in the test, when the type of solvent
is not specified, refers to aqueous solution.
A.2 Identification test
A.2.1 Color reaction of acetic anhydride concentrated sulfuric acid
A.2.1.1 Reagents and materials
A.2.1.1.1 Trichloromethane.
A.2.1.1.2 Acetic anhydride.
A.2.1.1.3 Sulfuric acid.
A.2.1.2 Identification method
Weigh 0.5mg of sample. Add 5mL of trichloromethane to dissolve. Add 0.3mL
of acetic anhydride and 0.1mL of sulfuric acid. Shake. Initially it is yellow. It
gradually becomes red. Then it immediately changes to purple, blue-green. It
finally turns green.
A.2.2 Infrared spectrum test
A.2.2.1 Reagents and materials
Potassium bromide.
A.2.2.2 Instruments and equipment
Infrared spectrometer.
A.2.2.3 Analysis steps
Use potassium bromide tablet method. Carry out the test according to GB/T
6040. The infrared spectrum of the sample shall be consistent with the standard
A.3.5 Analysis steps
A.3.5.1 Preparation of standard solution
Weigh 25mg of vitamin D3 standard product (to the nearest of 0.0001g). Place
in a 100mL brown volumetric flask. Add 80mL of isooctane. Avoid heating.
Perform ultrasonic treatment for 1min to make it completely dissolved. Use
isooctane to dilute to 100mL. Shake well. Use it as stock solution. Measure
5.0mL of the above solution. Place in a 50mL brown volumetric flask. Use
isooctane to dilute to the scale. Shake well. Use it as standard solution.
A.3.5.2 System suitability test
Measure 5.0mL of vitamin D3 stock solution (A.3.5.1) in a stoppered glass bottle.
Access to nitrogen then stopper it. Place in 90°C water bath to heat 1h. Take it
out to immediately cool. Add 5.0mL of n-ethane. Shake well. Place in a 1cm
plugged quartz absorption cell. Under two 8W UV lamps with dominant
wavelengths of 254nm and 365nm respectively. Place the quartz absorption
cell at an angle of 45°, 5cm ~ 6cm away from the light tube. Irradiate for 5min.
Make the solution contain vitamin D3, pre-vitamin D3, trans-vitamin D3 and
tachysterol D3. Measure the solution into the liquid chromatograph. Refer to
A.3.4 for chromatographic conditions. Conduct 5 sample injections. Record the
peak area. Calculate the relative standard deviation of vitamin D3 peak area not
more than 2.0%. The resolution of the pre-vitamin D3 peak and trans-vitamin D3
peak, as well as the vitamin D3 peak and tachysterol D3 peak shall be greater
than 1.0. The relative retention time of pre-vitamin D3, trans-vitamin D3,
tachysterol D3 and vitamin D3 are about 0.5, 0.6, 1.1, respectively. Analyze
under A.3.4 chromatographic conditions. The reference chromatogram is
shown in Figure C.1.
Weigh 25mg of 7-dehydrocholesterol standard product. Prepare 7-
dehydrocholesterol standard solution according to the preparation method of
standard solution (A.3.5.1). Respectively measure 2mL of Vitamin D3 standard
solution (A.3.5.1) and 7-dehydrocholesterol standard solution to prepare mixed
standard solution. Measure the solution into the liquid chromatograph. Refer to
A.3.4 for chromatographic conditions. Conduct 5 sample injections. Record the
peak area. Calculate the relative standard deviation of vitamin D3 peak area not
more than 2.0%. The resolution of the vitamin D3 peak and the 7-
dehydrocholesterol peak shall be greater than 1.0. The relative retention time
of 7-dehydrocholesterol and vitamin D3 is about 1.5. Analyze under A.3.4
chromatographic conditions. See Figure C.2 for reference chromatogram.
A.3.5.3 Preparation of sample solution
Weigh 25mg of vitamin D3 sample (to the nearest of 0.0001g). Place in a 100mL
brown volumetric flask. Add 80mL of isooctane. Avoid heating. Perform
A.5.4 Result calculation
The absorption coefficient, , is calculated according to
formula (A.3):
Where,
A - The value of the absorbance of the sample solution;
c - The mass fraction of the sample solution.
The absorbance value of the sample solution is based on the arithmetic average
of the two parallel determination results. The absolute difference between two
independent determination results obtained under repeated conditions is not
more than 2% of the arithmetic mean.
A.6 Relative substance
A.6.1 Reagents and materials
Same with A.3.2.
A.6.2 Instruments and equipment
Same with A.3.3.
A.6.3 Chromatographic reference conditions
Same with A.3.4.
A.6.4 Analysis steps
A.6.4.1 Preparation of sample solution
Weigh 25mg of vitamin D3 sample (to the nearest of 0.0001g). Place in a 100mL
brown volumetric flask. Add 80mL of isooctane. Avoid heating. Perform
ultrasonic treatment for 1min to make it completely dissolve. Use isooctane to
dilute to 100mL. Shake well and use it as the sample solution. Accurately
measure 1mL of the above solution. Place in a 100mL brown volumetric flask.
Use isooctane to dilute to the scale. Shake well and use it as the control solution.
A.6.4.2 System suitability test
Same with A.3.5.1, A.3.5.2.
GB 1903.50-2020
GB
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National food safety standard - Food nutritional
fortification substance - Cholecalciferol (Vitamin D3)
食品营养强化剂 胆钙化醇(维生素 D3)
ISSUED ON: SEPTEMBER 11, 2020
IMPLEMENTED ON: MARCH 11, 2021
Issued by: National Health Commission of the People's Republic of China;
State Administration for Market Regulation.
Table of Contents
1 Scope ... 3
2 Chemical name, structural formula, molecular formula, relative molecular
mass ... 3
3 Technical requirements ... 4
Annex A Inspection methods ... 5
Annex B Standard infrared spectrum of vitamin D3 ... 12
Annex C HPLC reference chromatogram of vitamin D3 and related substances
... 13
National food safety standard - Food nutritional
fortification substance - Cholecalciferol (Vitamin D3)
1 Scope
This Standard is applicable to food nutritional fortification substance -
cholecalciferol (Vitamin D3), which uses lanolin cholesterol as raw material, by
chemical synthesis, to obtain 7-dehydrocholesterol, then is made through UV
irradiation, refining and other processes.
2 Chemical name, structural formula, molecular
formula, relative molecular mass
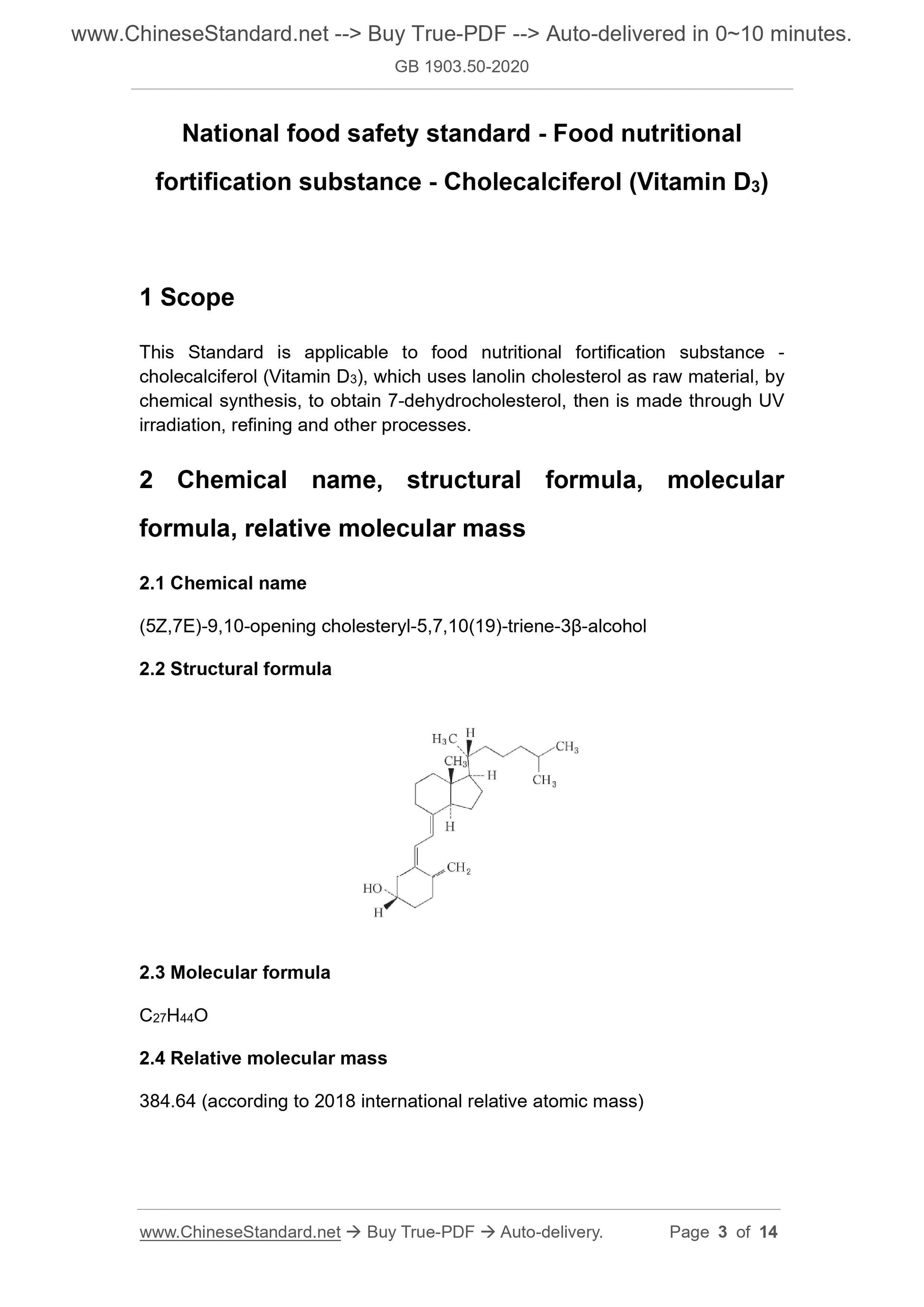
2.1 Chemical name
(5Z,7E)-9,10-opening cholesteryl-5,7,10(19)-triene-3β-alcohol
2.2 Structural formula
2.3 Molecular formula
C27H44O
2.4 Relative molecular mass
384.64 (according to 2018 international relative atomic mass)
Annex A
Inspection methods
A.1 General provisions
All reagents and water used in this Standard, when other requirements are not
specified, refer to analytically-pure reagents or of above specifications and
grade 3 water specified in GB/T 6682. Standard solutions used in the test,
standard solutions for impurity determination, preparations and products, when
other requirements are not specified, are prepared in accordance with GB/T
601, GB/T 602 and GB/T 603. Solution used in the test, when the type of solvent
is not specified, refers to aqueous solution.
A.2 Identification test
A.2.1 Color reaction of acetic anhydride concentrated sulfuric acid
A.2.1.1 Reagents and materials
A.2.1.1.1 Trichloromethane.
A.2.1.1.2 Acetic anhydride.
A.2.1.1.3 Sulfuric acid.
A.2.1.2 Identification method
Weigh 0.5mg of sample. Add 5mL of trichloromethane to dissolve. Add 0.3mL
of acetic anhydride and 0.1mL of sulfuric acid. Shake. Initially it is yellow. It
gradually becomes red. Then it immediately changes to purple, blue-green. It
finally turns green.
A.2.2 Infrared spectrum test
A.2.2.1 Reagents and materials
Potassium bromide.
A.2.2.2 Instruments and equipment
Infrared spectrometer.
A.2.2.3 Analysis steps
Use potassium bromide tablet method. Carry out the test according to GB/T
6040. The infrared spectrum of the sample shall be consistent with the standard
A.3.5 Analysis steps
A.3.5.1 Preparation of standard solution
Weigh 25mg of vitamin D3 standard product (to the nearest of 0.0001g). Place
in a 100mL brown volumetric flask. Add 80mL of isooctane. Avoid heating.
Perform ultrasonic treatment for 1min to make it completely dissolved. Use
isooctane to dilute to 100mL. Shake well. Use it as stock solution. Measure
5.0mL of the above solution. Place in a 50mL brown volumetric flask. Use
isooctane to dilute to the scale. Shake well. Use it as standard solution.
A.3.5.2 System suitability test
Measure 5.0mL of vitamin D3 stock solution (A.3.5.1) in a stoppered glass bottle.
Access to nitrogen then stopper it. Place in 90°C water bath to heat 1h. Take it
out to immediately cool. Add 5.0mL of n-ethane. Shake well. Place in a 1cm
plugged quartz absorption cell. Under two 8W UV lamps with dominant
wavelengths of 254nm and 365nm respectively. Place the quartz absorption
cell at an angle of 45°, 5cm ~ 6cm away from the light tube. Irradiate for 5min.
Make the solution contain vitamin D3, pre-vitamin D3, trans-vitamin D3 and
tachysterol D3. Measure the solution into the liquid chromatograph. Refer to
A.3.4 for chromatographic conditions. Conduct 5 sample injections. Record the
peak area. Calculate the relative standard deviation of vitamin D3 peak area not
more than 2.0%. The resolution of the pre-vitamin D3 peak and trans-vitamin D3
peak, as well as the vitamin D3 peak and tachysterol D3 peak shall be greater
than 1.0. The relative retention time of pre-vitamin D3, trans-vitamin D3,
tachysterol D3 and vitamin D3 are about 0.5, 0.6, 1.1, respectively. Analyze
under A.3.4 chromatographic conditions. The reference chromatogram is
shown in Figure C.1.
Weigh 25mg of 7-dehydrocholesterol standard product. Prepare 7-
dehydrocholesterol standard solution according to the preparation method of
standard solution (A.3.5.1). Respectively measure 2mL of Vitamin D3 standard
solution (A.3.5.1) and 7-dehydrocholesterol standard solution to prepare mixed
standard solution. Measure the solution into the liquid chromatograph. Refer to
A.3.4 for chromatographic conditions. Conduct 5 sample injections. Record the
peak area. Calculate the relative standard deviation of vitamin D3 peak area not
more than 2.0%. The resolution of the vitamin D3 peak and the 7-
dehydrocholesterol peak shall be greater than 1.0. The relative retention time
of 7-dehydrocholesterol and vitamin D3 is about 1.5. Analyze under A.3.4
chromatographic conditions. See Figure C.2 for reference chromatogram.
A.3.5.3 Preparation of sample solution
Weigh 25mg of vitamin D3 sample (to the nearest of 0.0001g). Place in a 100mL
brown volumetric flask. Add 80mL of isooctane. Avoid heating. Perform
A.5.4 Result calculation
The absorption coefficient, , is calculated according to
formula (A.3):
Where,
A - The value of the absorbance of the sample solution;
c - The mass fraction of the sample solution.
The absorbance value of the sample solution is based on the arithmetic average
of the two parallel determination results. The absolute difference between two
independent determination results obtained under repeated conditions is not
more than 2% of the arithmetic mean.
A.6 Relative substance
A.6.1 Reagents and materials
Same with A.3.2.
A.6.2 Instruments and equipment
Same with A.3.3.
A.6.3 Chromatographic reference conditions
Same with A.3.4.
A.6.4 Analysis steps
A.6.4.1 Preparation of sample solution
Weigh 25mg of vitamin D3 sample (to the nearest of 0.0001g). Place in a 100mL
brown volumetric flask. Add 80mL of isooctane. Avoid heating. Perform
ultrasonic treatment for 1min to make it completely dissolve. Use isooctane to
dilute to 100mL. Shake well and use it as the sample solution. Accurately
measure 1mL of the above solution. Place in a 100mL brown volumetric flask.
Use isooctane to dilute to the scale. Shake well and use it as the control solution.
A.6.4.2 System suitability test
Same with A.3.5.1, A.3.5.2.
Delivery: 9 seconds. Download (& Email) true-PDF + Invoice.
Get Quotation: Click GB 1903.50-2020 (Self-service in 1-minute)
Historical versions (Master-website): GB 1903.50-2020
Preview True-PDF (Reload/Scroll-down if blank)
GB 1903.50-2020
GB
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National food safety standard - Food nutritional
fortification substance - Cholecalciferol (Vitamin D3)
食品营养强化剂 胆钙化醇(维生素 D3)
ISSUED ON: SEPTEMBER 11, 2020
IMPLEMENTED ON: MARCH 11, 2021
Issued by: National Health Commission of the People's Republic of China;
State Administration for Market Regulation.
Table of Contents
1 Scope ... 3
2 Chemical name, structural formula, molecular formula, relative molecular
mass ... 3
3 Technical requirements ... 4
Annex A Inspection methods ... 5
Annex B Standard infrared spectrum of vitamin D3 ... 12
Annex C HPLC reference chromatogram of vitamin D3 and related substances
... 13
National food safety standard - Food nutritional
fortification substance - Cholecalciferol (Vitamin D3)
1 Scope
This Standard is applicable to food nutritional fortification substance -
cholecalciferol (Vitamin D3), which uses lanolin cholesterol as raw material, by
chemical synthesis, to obtain 7-dehydrocholesterol, then is made through UV
irradiation, refining and other processes.
2 Chemical name, structural formula, molecular
formula, relative molecular mass
2.1 Chemical name
(5Z,7E)-9,10-opening cholesteryl-5,7,10(19)-triene-3β-alcohol
2.2 Structural formula
2.3 Molecular formula
C27H44O
2.4 Relative molecular mass
384.64 (according to 2018 international relative atomic mass)
Annex A
Inspection methods
A.1 General provisions
All reagents and water used in this Standard, when other requirements are not
specified, refer to analytically-pure reagents or of above specifications and
grade 3 water specified in GB/T 6682. Standard solutions used in the test,
standard solutions for impurity determination, preparations and products, when
other requirements are not specified, are prepared in accordance with GB/T
601, GB/T 602 and GB/T 603. Solution used in the test, when the type of solvent
is not specified, refers to aqueous solution.
A.2 Identification test
A.2.1 Color reaction of acetic anhydride concentrated sulfuric acid
A.2.1.1 Reagents and materials
A.2.1.1.1 Trichloromethane.
A.2.1.1.2 Acetic anhydride.
A.2.1.1.3 Sulfuric acid.
A.2.1.2 Identification method
Weigh 0.5mg of sample. Add 5mL of trichloromethane to dissolve. Add 0.3mL
of acetic anhydride and 0.1mL of sulfuric acid. Shake. Initially it is yellow. It
gradually becomes red. Then it immediately changes to purple, blue-green. It
finally turns green.
A.2.2 Infrared spectrum test
A.2.2.1 Reagents and materials
Potassium bromide.
A.2.2.2 Instruments and equipment
Infrared spectrometer.
A.2.2.3 Analysis steps
Use potassium bromide tablet method. Carry out the test according to GB/T
6040. The infrared spectrum of the sample shall be consistent with the standard
A.3.5 Analysis steps
A.3.5.1 Preparation of standard solution
Weigh 25mg of vitamin D3 standard product (to the nearest of 0.0001g). Place
in a 100mL brown volumetric flask. Add 80mL of isooctane. Avoid heating.
Perform ultrasonic treatment for 1min to make it completely dissolved. Use
isooctane to dilute to 100mL. Shake well. Use it as stock solution. Measure
5.0mL of the above solution. Place in a 50mL brown volumetric flask. Use
isooctane to dilute to the scale. Shake well. Use it as standard solution.
A.3.5.2 System suitability test
Measure 5.0mL of vitamin D3 stock solution (A.3.5.1) in a stoppered glass bottle.
Access to nitrogen then stopper it. Place in 90°C water bath to heat 1h. Take it
out to immediately cool. Add 5.0mL of n-ethane. Shake well. Place in a 1cm
plugged quartz absorption cell. Under two 8W UV lamps with dominant
wavelengths of 254nm and 365nm respectively. Place the quartz absorption
cell at an angle of 45°, 5cm ~ 6cm away from the light tube. Irradiate for 5min.
Make the solution contain vitamin D3, pre-vitamin D3, trans-vitamin D3 and
tachysterol D3. Measure the solution into the liquid chromatograph. Refer to
A.3.4 for chromatographic conditions. Conduct 5 sample injections. Record the
peak area. Calculate the relative standard deviation of vitamin D3 peak area not
more than 2.0%. The resolution of the pre-vitamin D3 peak and trans-vitamin D3
peak, as well as the vitamin D3 peak and tachysterol D3 peak shall be greater
than 1.0. The relative retention time of pre-vitamin D3, trans-vitamin D3,
tachysterol D3 and vitamin D3 are about 0.5, 0.6, 1.1, respectively. Analyze
under A.3.4 chromatographic conditions. The reference chromatogram is
shown in Figure C.1.
Weigh 25mg of 7-dehydrocholesterol standard product. Prepare 7-
dehydrocholesterol standard solution according to the preparation method of
standard solution (A.3.5.1). Respectively measure 2mL of Vitamin D3 standard
solution (A.3.5.1) and 7-dehydrocholesterol standard solution to prepare mixed
standard solution. Measure the solution into the liquid chromatograph. Refer to
A.3.4 for chromatographic conditions. Conduct 5 sample injections. Record the
peak area. Calculate the relative standard deviation of vitamin D3 peak area not
more than 2.0%. The resolution of the vitamin D3 peak and the 7-
dehydrocholesterol peak shall be greater than 1.0. The relative retention time
of 7-dehydrocholesterol and vitamin D3 is about 1.5. Analyze under A.3.4
chromatographic conditions. See Figure C.2 for reference chromatogram.
A.3.5.3 Preparation of sample solution
Weigh 25mg of vitamin D3 sample (to the nearest of 0.0001g). Place in a 100mL
brown volumetric flask. Add 80mL of isooctane. Avoid heating. Perform
A.5.4 Result calculation
The absorption coefficient, , is calculated according to
formula (A.3):
Where,
A - The value of the absorbance of the sample solution;
c - The mass fraction of the sample solution.
The absorbance value of the sample solution is based on the arithmetic average
of the two parallel determination results. The absolute difference between two
independent determination results obtained under repeated conditions is not
more than 2% of the arithmetic mean.
A.6 Relative substance
A.6.1 Reagents and materials
Same with A.3.2.
A.6.2 Instruments and equipment
Same with A.3.3.
A.6.3 Chromatographic reference conditions
Same with A.3.4.
A.6.4 Analysis steps
A.6.4.1 Preparation of sample solution
Weigh 25mg of vitamin D3 sample (to the nearest of 0.0001g). Place in a 100mL
brown volumetric flask. Add 80mL of isooctane. Avoid heating. Perform
ultrasonic treatment for 1min to make it completely dissolve. Use isooctane to
dilute to 100mL. Shake well and use it as the sample solution. Accurately
measure 1mL of the above solution. Place in a 100mL brown volumetric flask.
Use isooctane to dilute to the scale. Shake well and use it as the control solution.
A.6.4.2 System suitability test
Same with A.3.5.1, A.3.5.2.
GB 1903.50-2020
GB
NATIONAL STANDARD OF THE
PEOPLE’S REPUBLIC OF CHINA
National food safety standard - Food nutritional
fortification substance - Cholecalciferol (Vitamin D3)
食品营养强化剂 胆钙化醇(维生素 D3)
ISSUED ON: SEPTEMBER 11, 2020
IMPLEMENTED ON: MARCH 11, 2021
Issued by: National Health Commission of the People's Republic of China;
State Administration for Market Regulation.
Table of Contents
1 Scope ... 3
2 Chemical name, structural formula, molecular formula, relative molecular
mass ... 3
3 Technical requirements ... 4
Annex A Inspection methods ... 5
Annex B Standard infrared spectrum of vitamin D3 ... 12
Annex C HPLC reference chromatogram of vitamin D3 and related substances
... 13
National food safety standard - Food nutritional
fortification substance - Cholecalciferol (Vitamin D3)
1 Scope
This Standard is applicable to food nutritional fortification substance -
cholecalciferol (Vitamin D3), which uses lanolin cholesterol as raw material, by
chemical synthesis, to obtain 7-dehydrocholesterol, then is made through UV
irradiation, refining and other processes.
2 Chemical name, structural formula, molecular
formula, relative molecular mass
2.1 Chemical name
(5Z,7E)-9,10-opening cholesteryl-5,7,10(19)-triene-3β-alcohol
2.2 Structural formula
2.3 Molecular formula
C27H44O
2.4 Relative molecular mass
384.64 (according to 2018 international relative atomic mass)
Annex A
Inspection methods
A.1 General provisions
All reagents and water used in this Standard, when other requirements are not
specified, refer to analytically-pure reagents or of above specifications and
grade 3 water specified in GB/T 6682. Standard solutions used in the test,
standard solutions for impurity determination, preparations and products, when
other requirements are not specified, are prepared in accordance with GB/T
601, GB/T 602 and GB/T 603. Solution used in the test, when the type of solvent
is not specified, refers to aqueous solution.
A.2 Identification test
A.2.1 Color reaction of acetic anhydride concentrated sulfuric acid
A.2.1.1 Reagents and materials
A.2.1.1.1 Trichloromethane.
A.2.1.1.2 Acetic anhydride.
A.2.1.1.3 Sulfuric acid.
A.2.1.2 Identification method
Weigh 0.5mg of sample. Add 5mL of trichloromethane to dissolve. Add 0.3mL
of acetic anhydride and 0.1mL of sulfuric acid. Shake. Initially it is yellow. It
gradually becomes red. Then it immediately changes to purple, blue-green. It
finally turns green.
A.2.2 Infrared spectrum test
A.2.2.1 Reagents and materials
Potassium bromide.
A.2.2.2 Instruments and equipment
Infrared spectrometer.
A.2.2.3 Analysis steps
Use potassium bromide tablet method. Carry out the test according to GB/T
6040. The infrared spectrum of the sample shall be consistent with the standard
A.3.5 Analysis steps
A.3.5.1 Preparation of standard solution
Weigh 25mg of vitamin D3 standard product (to the nearest of 0.0001g). Place
in a 100mL brown volumetric flask. Add 80mL of isooctane. Avoid heating.
Perform ultrasonic treatment for 1min to make it completely dissolved. Use
isooctane to dilute to 100mL. Shake well. Use it as stock solution. Measure
5.0mL of the above solution. Place in a 50mL brown volumetric flask. Use
isooctane to dilute to the scale. Shake well. Use it as standard solution.
A.3.5.2 System suitability test
Measure 5.0mL of vitamin D3 stock solution (A.3.5.1) in a stoppered glass bottle.
Access to nitrogen then stopper it. Place in 90°C water bath to heat 1h. Take it
out to immediately cool. Add 5.0mL of n-ethane. Shake well. Place in a 1cm
plugged quartz absorption cell. Under two 8W UV lamps with dominant
wavelengths of 254nm and 365nm respectively. Place the quartz absorption
cell at an angle of 45°, 5cm ~ 6cm away from the light tube. Irradiate for 5min.
Make the solution contain vitamin D3, pre-vitamin D3, trans-vitamin D3 and
tachysterol D3. Measure the solution into the liquid chromatograph. Refer to
A.3.4 for chromatographic conditions. Conduct 5 sample injections. Record the
peak area. Calculate the relative standard deviation of vitamin D3 peak area not
more than 2.0%. The resolution of the pre-vitamin D3 peak and trans-vitamin D3
peak, as well as the vitamin D3 peak and tachysterol D3 peak shall be greater
than 1.0. The relative retention time of pre-vitamin D3, trans-vitamin D3,
tachysterol D3 and vitamin D3 are about 0.5, 0.6, 1.1, respectively. Analyze
under A.3.4 chromatographic conditions. The reference chromatogram is
shown in Figure C.1.
Weigh 25mg of 7-dehydrocholesterol standard product. Prepare 7-
dehydrocholesterol standard solution according to the preparation method of
standard solution (A.3.5.1). Respectively measure 2mL of Vitamin D3 standard
solution (A.3.5.1) and 7-dehydrocholesterol standard solution to prepare mixed
standard solution. Measure the solution into the liquid chromatograph. Refer to
A.3.4 for chromatographic conditions. Conduct 5 sample injections. Record the
peak area. Calculate the relative standard deviation of vitamin D3 peak area not
more than 2.0%. The resolution of the vitamin D3 peak and the 7-
dehydrocholesterol peak shall be greater than 1.0. The relative retention time
of 7-dehydrocholesterol and vitamin D3 is about 1.5. Analyze under A.3.4
chromatographic conditions. See Figure C.2 for reference chromatogram.
A.3.5.3 Preparation of sample solution
Weigh 25mg of vitamin D3 sample (to the nearest of 0.0001g). Place in a 100mL
brown volumetric flask. Add 80mL of isooctane. Avoid heating. Perform
A.5.4 Result calculation
The absorption coefficient, , is calculated according to
formula (A.3):
Where,
A - The value of the absorbance of the sample solution;
c - The mass fraction of the sample solution.
The absorbance value of the sample solution is based on the arithmetic average
of the two parallel determination results. The absolute difference between two
independent determination results obtained under repeated conditions is not
more than 2% of the arithmetic mean.
A.6 Relative substance
A.6.1 Reagents and materials
Same with A.3.2.
A.6.2 Instruments and equipment
Same with A.3.3.
A.6.3 Chromatographic reference conditions
Same with A.3.4.
A.6.4 Analysis steps
A.6.4.1 Preparation of sample solution
Weigh 25mg of vitamin D3 sample (to the nearest of 0.0001g). Place in a 100mL
brown volumetric flask. Add 80mL of isooctane. Avoid heating. Perform
ultrasonic treatment for 1min to make it completely dissolve. Use isooctane to
dilute to 100mL. Shake well and use it as the sample solution. Accurately
measure 1mL of the above solution. Place in a 100mL brown volumetric flask.
Use isooctane to dilute to the scale. Shake well and use it as the control solution.
A.6.4.2 System suitability test
Same with A.3.5.1, A.3.5.2.
Share