1
/
of
9
PayPal, credit cards. Download editable-PDF & invoice In 1 second!
GB 4789.9-2014 English PDF (GB4789.9-2014)
GB 4789.9-2014 English PDF (GB4789.9-2014)
Regular price
$130.00 USD
Regular price
Sale price
$130.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click GB 4789.9-2014
Historical versions: GB 4789.9-2014
Preview True-PDF (Reload/Scroll if blank)
GB 4789.9-2014: Microbiological examination of food hygiene -- Examination of Campylobacter jejuni
GB 4789.9-2014
GB
NATIONAL FOOD SAFETY STANDARD OF
THE PEOPLE’S REPUBLIC OF CHINA
GB/T 4789.9-2014
Microbiological examination of food hygiene -
Examination of Campylobacter jejuni
ISSUED ON. DECEMBER 1, 2014
IMPLEMENTED ON. MAY 1, 2015
Issued by. National Health and Family Planning Commission of the
People 's Republic of China
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Equipment and materials ... 4
3 Media and reagents ... 5
4 Examination procedures ... 5
5 Operation steps ... 6
Annex A Media and reagents ... 11
Foreword
This Standard replaces GB/T 4789.9-2008 Microbiological examination of food
hygiene - Examination of Campylobacter jejuni.
Compared with GB/T 4789.9-2008, the main changes of this Standard are as
follows.
- modified the Chinese name of the standard;
- modified the scope;
- modified the equipment and material;
- modified the media and reagents;
- modified sample treatment;
- deleted the drug sensitivity test;
- deleted Method 2 - Automatic enzyme - linked immunosorbent assay.
Microbiological examination of food hygiene -
Examination of Campylobacter jejuni
1 Scope
This Standard specifies the examination method of Campylobacter jejuni in
food.
This Standard is applicable to the examination of Campylobacter jejuni in food.
2 Equipment and materials
In addition to microbiology laboratory routine sterilization and culture equipment,
other equipment and materials are as follows.
a) constant temperature incubator. 25°C ± 1°C, 36°C ± 1°C, 42°C ± 1°C;
b) refrigerator. 2°C ~ 5°C;
c) constant temperature oscillation incubator. 36°C ± 1°C, 42°C ± 1°C;
d) balance. a sense of 0.1g;
e) homogenizer and matching homogeneous bag;
f) oscillator;
g) sterile pipette. 1 mL (with 0.01 mL scale), 10 mL (with 0.1 mL scale) or
micropipette and tip;
h) sterile Erlenmeyer flask. capacity of 100 mL, 200 mL, 2000 mL;
i) sterile petri dish. 90 mm in diameter;
j) pH meter or pH colorimetric tube or precision pH test paper;
k) water bath device. 36°C ± 1°C, 100°C;
l) micro-aerobic incubator. providing micro-aerobic conditions (5% oxygen,
10% carbon dioxide and 85% nitrogen);
m) filter device and filter membrane (0.22 µm, 0.45 µm);
5.1.1 General sample
Take 25 g (mL) of sample (50 g for fruits, vegetables, aquatic products) into the
homogeneous bag with screen filled with 225 mL of Bolton broth (it may use
sterile gauze for filtration if there is no homogeneous bag with screen). Use
rapping homogenizer to homogenize for 1 min ~ 2 min. Filter it through strainer
or sterile gauze. Cultivate the filtrate.
5.1.2 Whole poultry and other samples
Use 200 mL of 0.1% peptone water to rinse the inside and outside of the sample
sufficiently. Oscillate it for 2 min ~ 3 min. Filter it through sterile gauze into a
250 mL centrifuge tube. After 16000 g is centrifuged for 15 min, discard the
supernatant liquid. Use 10 mL of 0.1% peptone water to conduct suspension
precipitation. Pipet 3 mL into 100 mL of Bolton broth to cultivate.
5.1.3 Shellfish
Table at least 12 samples with shell. After removal of the housing, all contents
are put into a homogeneous bag. Use rapping homogenizer to homogenize for
1 min ~ 2 min. Take 25 g of sample into 225 mL of Bolton broth (1.10 dilution).
After sufficient shaking, transfer 25 mL to 225 mL of Bolton broth (1.100 dilution).
Cultivate the Bolton broth diluted in 1.10 and the Bolton broth diluted in 1.100
simultaneously.
5.1.4 Egg yolk liquid or egg slurry
Take 25 g (mL) of sample into 125 mL of Bolton broth and well mix it (1.6
dilution). Transfer 25 mL into 100 mL of Bolton broth and well mix it (1.30
dilution). Cultivate the Bolton broth diluted in 1.6 and the Bolton broth diluted in
1.30 simultaneously.
5.1.5 Fresh milk, ice cream, cheese, etc.
If it is liquid dairy product, take 50 g. If it is solid dairy product, take 50 g into the
homogeneous bag with screen filled with 50 mL of 0.1% peptone water. Use
rapping homogenizer to homogenize for 15 s ~ 30 s. Retain the filtrate. When
necessary, adjust pH to 7.5 ± 0.2. After 20000 g of liquid diary product or filtrate
is centrifuged for 30 min, discard the supernatant liquid. Use 10 mL of Bolton
broth to conduct suspension precipitation (try to avoid into the reservoir).
Transfer to 90 mL of Bolton broth to cultivate.
5.1.6 Samples to be smeared on the surface
Use sterile cotton swab to wipe the surface of the test sample (the area is at
least greater than 100 cm2). Cut the cotton swab head into 100 mL of Bolton
broth to cultivate.
5.4.2.2 Sodium hippurate hydrolysis test
Pick colonies. Add into a test tube filled with 0.4 mL of 1% sodium hippurate to
make bacterial suspension. After mixing evenly, incubate at 36°C ± 1°C water
bath for 2 h or 36°C ± 1°C incubator for 4 h. Along with the tube wall, slowly add
into 0.2 mL of ninhydrin solution. Do not oscillate. Incubate at 36°C ± 1°C water
bath or incubator for another 10 min. Then read the results. If it appears purple,
it shall be positive. If it appears light purple or there is no color change, it shall
be negative.
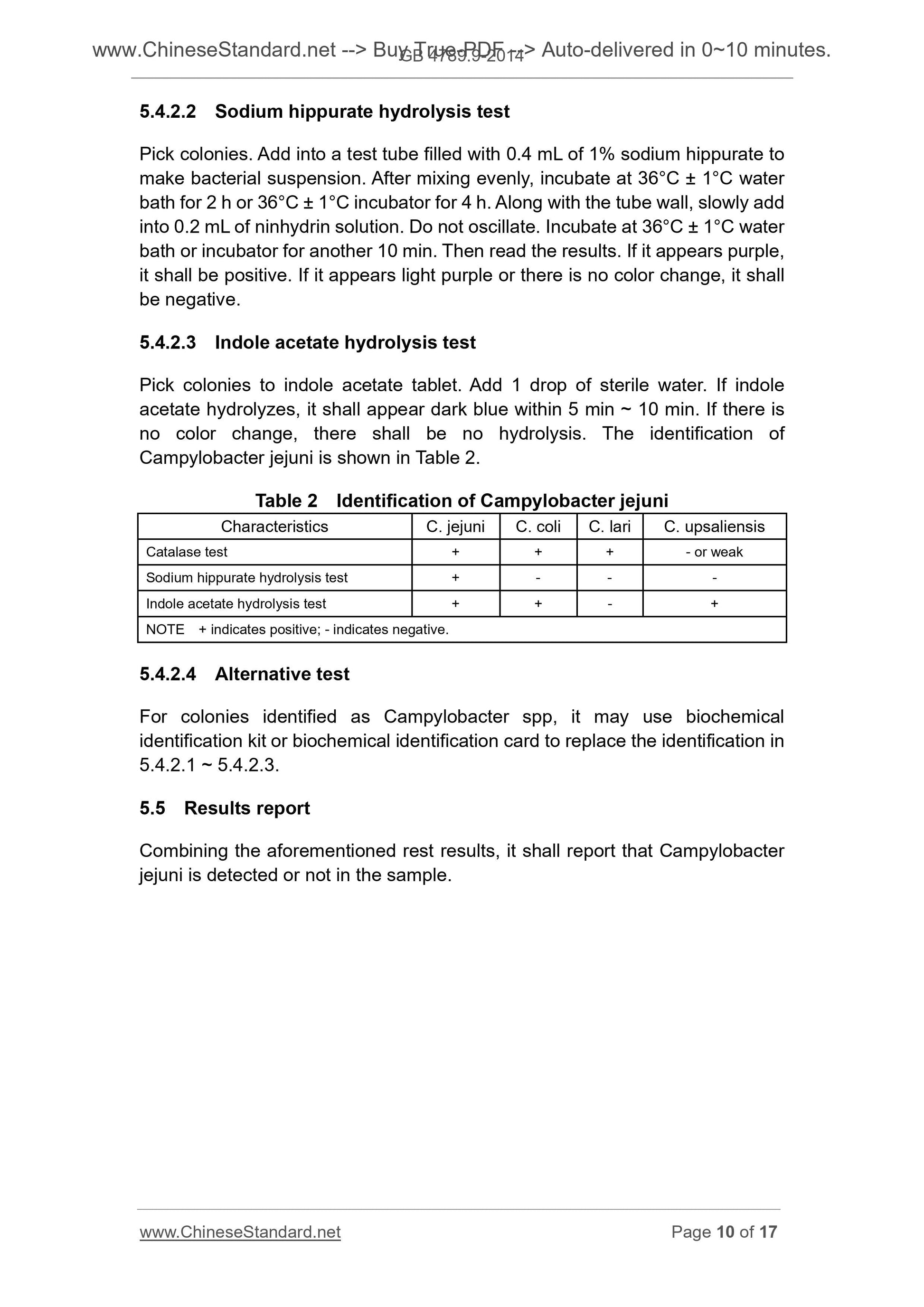
5.4.2.3 Indole acetate hydrolysis test
Pick colonies to indole acetate tablet. Add 1 drop of sterile water. If indole
acetate hydrolyzes, it shall appear dark blue within 5 min ~ 10 min. If there is
no color change, there shall be no hydrolysis. The identification of
Campylobacter jejuni is shown in Table 2.
Table 2 Identification of Campylobacter jejuni
Characteristics C. jejuni C. coli C. lari C. upsaliensis
Catalase test + + + - or weak
Sodium hippurate hydrolysis test + - - -
Indole acetate hydrolysis test + + - +
NOTE + indicates positive; - indicates negative.
5.4.2.4 Alternative test
For colonies identified as Campylobacter spp, it may use biochemical
identification kit or biochemical identification card to replace the identification in
5.4.2.1 ~ 5.4.2.3.
5.5 Results report
Combining the aforementioned rest results, it shall report that Campylobacter
jejuni is detected or not in the sample.
A.1.4.1 Composition
Basal medium 1000.0 mL
Sterile lysis defibrillation sheep or horse blood 50.0 mL
Antibiotic solution 5.0 mL
A.1.4.2 Preparation method
When the basal medium temperature is around 45°C, sterilely add sheep or
horse blood and antibiotic solution. Well mix. Calibrate pH to 7.4 ± 0.2 (25°C).
Placement at room temperature shall not exceed 4 h. Or storage in darkness at
4°C must not exceed 7 d.
A.2 modified Charcoal Cefoperazone Deoxycholate Agar, mCCDA
A.2.1 Basal medium
A.2.1.1 Composition
Meat infusion 10.0 g
Animal tissue hydrolyzate 10.0 g
Sodium chloride 5.0 g
Charcoal 4.0 g
Casein hydrolyzate 3.0 g
Sodium deoxycholate 1.0 g
Ferrous sulfate 0.25 g
Sodium pyruvate 0.25 g
Agar 8.0 g ~ 18.0 g
Distilled water 1000.0 mL
A.2.1.2 Preparation method
Dissolve the compositions in A.2.1.1 into distilled water. Sterilize at 121°C for
15 min, ready for use.
A.2.2 Antibiotic solution
A.2.2.1 Composition
cefoperazone 0.032 g
amphotericin B 0.01 g
rifampicin 0.01 g
ethanol / sterile water (50 / 50, volume fraction) 5.0 mL
A.2.2.2 Preparation method
Dissolve the compositions in A.2.2.1 into ethanol / sterile water mixed solution.
medium into sterile plate. Place still till medium is solidified. When the plate for
preparation is not dry, placement at room temperature shall not exceed...
Get QUOTATION in 1-minute: Click GB 4789.9-2014
Historical versions: GB 4789.9-2014
Preview True-PDF (Reload/Scroll if blank)
GB 4789.9-2014: Microbiological examination of food hygiene -- Examination of Campylobacter jejuni
GB 4789.9-2014
GB
NATIONAL FOOD SAFETY STANDARD OF
THE PEOPLE’S REPUBLIC OF CHINA
GB/T 4789.9-2014
Microbiological examination of food hygiene -
Examination of Campylobacter jejuni
ISSUED ON. DECEMBER 1, 2014
IMPLEMENTED ON. MAY 1, 2015
Issued by. National Health and Family Planning Commission of the
People 's Republic of China
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Equipment and materials ... 4
3 Media and reagents ... 5
4 Examination procedures ... 5
5 Operation steps ... 6
Annex A Media and reagents ... 11
Foreword
This Standard replaces GB/T 4789.9-2008 Microbiological examination of food
hygiene - Examination of Campylobacter jejuni.
Compared with GB/T 4789.9-2008, the main changes of this Standard are as
follows.
- modified the Chinese name of the standard;
- modified the scope;
- modified the equipment and material;
- modified the media and reagents;
- modified sample treatment;
- deleted the drug sensitivity test;
- deleted Method 2 - Automatic enzyme - linked immunosorbent assay.
Microbiological examination of food hygiene -
Examination of Campylobacter jejuni
1 Scope
This Standard specifies the examination method of Campylobacter jejuni in
food.
This Standard is applicable to the examination of Campylobacter jejuni in food.
2 Equipment and materials
In addition to microbiology laboratory routine sterilization and culture equipment,
other equipment and materials are as follows.
a) constant temperature incubator. 25°C ± 1°C, 36°C ± 1°C, 42°C ± 1°C;
b) refrigerator. 2°C ~ 5°C;
c) constant temperature oscillation incubator. 36°C ± 1°C, 42°C ± 1°C;
d) balance. a sense of 0.1g;
e) homogenizer and matching homogeneous bag;
f) oscillator;
g) sterile pipette. 1 mL (with 0.01 mL scale), 10 mL (with 0.1 mL scale) or
micropipette and tip;
h) sterile Erlenmeyer flask. capacity of 100 mL, 200 mL, 2000 mL;
i) sterile petri dish. 90 mm in diameter;
j) pH meter or pH colorimetric tube or precision pH test paper;
k) water bath device. 36°C ± 1°C, 100°C;
l) micro-aerobic incubator. providing micro-aerobic conditions (5% oxygen,
10% carbon dioxide and 85% nitrogen);
m) filter device and filter membrane (0.22 µm, 0.45 µm);
5.1.1 General sample
Take 25 g (mL) of sample (50 g for fruits, vegetables, aquatic products) into the
homogeneous bag with screen filled with 225 mL of Bolton broth (it may use
sterile gauze for filtration if there is no homogeneous bag with screen). Use
rapping homogenizer to homogenize for 1 min ~ 2 min. Filter it through strainer
or sterile gauze. Cultivate the filtrate.
5.1.2 Whole poultry and other samples
Use 200 mL of 0.1% peptone water to rinse the inside and outside of the sample
sufficiently. Oscillate it for 2 min ~ 3 min. Filter it through sterile gauze into a
250 mL centrifuge tube. After 16000 g is centrifuged for 15 min, discard the
supernatant liquid. Use 10 mL of 0.1% peptone water to conduct suspension
precipitation. Pipet 3 mL into 100 mL of Bolton broth to cultivate.
5.1.3 Shellfish
Table at least 12 samples with shell. After removal of the housing, all contents
are put into a homogeneous bag. Use rapping homogenizer to homogenize for
1 min ~ 2 min. Take 25 g of sample into 225 mL of Bolton broth (1.10 dilution).
After sufficient shaking, transfer 25 mL to 225 mL of Bolton broth (1.100 dilution).
Cultivate the Bolton broth diluted in 1.10 and the Bolton broth diluted in 1.100
simultaneously.
5.1.4 Egg yolk liquid or egg slurry
Take 25 g (mL) of sample into 125 mL of Bolton broth and well mix it (1.6
dilution). Transfer 25 mL into 100 mL of Bolton broth and well mix it (1.30
dilution). Cultivate the Bolton broth diluted in 1.6 and the Bolton broth diluted in
1.30 simultaneously.
5.1.5 Fresh milk, ice cream, cheese, etc.
If it is liquid dairy product, take 50 g. If it is solid dairy product, take 50 g into the
homogeneous bag with screen filled with 50 mL of 0.1% peptone water. Use
rapping homogenizer to homogenize for 15 s ~ 30 s. Retain the filtrate. When
necessary, adjust pH to 7.5 ± 0.2. After 20000 g of liquid diary product or filtrate
is centrifuged for 30 min, discard the supernatant liquid. Use 10 mL of Bolton
broth to conduct suspension precipitation (try to avoid into the reservoir).
Transfer to 90 mL of Bolton broth to cultivate.
5.1.6 Samples to be smeared on the surface
Use sterile cotton swab to wipe the surface of the test sample (the area is at
least greater than 100 cm2). Cut the cotton swab head into 100 mL of Bolton
broth to cultivate.
5.4.2.2 Sodium hippurate hydrolysis test
Pick colonies. Add into a test tube filled with 0.4 mL of 1% sodium hippurate to
make bacterial suspension. After mixing evenly, incubate at 36°C ± 1°C water
bath for 2 h or 36°C ± 1°C incubator for 4 h. Along with the tube wall, slowly add
into 0.2 mL of ninhydrin solution. Do not oscillate. Incubate at 36°C ± 1°C water
bath or incubator for another 10 min. Then read the results. If it appears purple,
it shall be positive. If it appears light purple or there is no color change, it shall
be negative.
5.4.2.3 Indole acetate hydrolysis test
Pick colonies to indole acetate tablet. Add 1 drop of sterile water. If indole
acetate hydrolyzes, it shall appear dark blue within 5 min ~ 10 min. If there is
no color change, there shall be no hydrolysis. The identification of
Campylobacter jejuni is shown in Table 2.
Table 2 Identification of Campylobacter jejuni
Characteristics C. jejuni C. coli C. lari C. upsaliensis
Catalase test + + + - or weak
Sodium hippurate hydrolysis test + - - -
Indole acetate hydrolysis test + + - +
NOTE + indicates positive; - indicates negative.
5.4.2.4 Alternative test
For colonies identified as Campylobacter spp, it may use biochemical
identification kit or biochemical identification card to replace the identification in
5.4.2.1 ~ 5.4.2.3.
5.5 Results report
Combining the aforementioned rest results, it shall report that Campylobacter
jejuni is detected or not in the sample.
A.1.4.1 Composition
Basal medium 1000.0 mL
Sterile lysis defibrillation sheep or horse blood 50.0 mL
Antibiotic solution 5.0 mL
A.1.4.2 Preparation method
When the basal medium temperature is around 45°C, sterilely add sheep or
horse blood and antibiotic solution. Well mix. Calibrate pH to 7.4 ± 0.2 (25°C).
Placement at room temperature shall not exceed 4 h. Or storage in darkness at
4°C must not exceed 7 d.
A.2 modified Charcoal Cefoperazone Deoxycholate Agar, mCCDA
A.2.1 Basal medium
A.2.1.1 Composition
Meat infusion 10.0 g
Animal tissue hydrolyzate 10.0 g
Sodium chloride 5.0 g
Charcoal 4.0 g
Casein hydrolyzate 3.0 g
Sodium deoxycholate 1.0 g
Ferrous sulfate 0.25 g
Sodium pyruvate 0.25 g
Agar 8.0 g ~ 18.0 g
Distilled water 1000.0 mL
A.2.1.2 Preparation method
Dissolve the compositions in A.2.1.1 into distilled water. Sterilize at 121°C for
15 min, ready for use.
A.2.2 Antibiotic solution
A.2.2.1 Composition
cefoperazone 0.032 g
amphotericin B 0.01 g
rifampicin 0.01 g
ethanol / sterile water (50 / 50, volume fraction) 5.0 mL
A.2.2.2 Preparation method
Dissolve the compositions in A.2.2.1 into ethanol / sterile water mixed solution.
medium into sterile plate. Place still till medium is solidified. When the plate for
preparation is not dry, placement at room temperature shall not exceed...
Share