1
/
of
6
www.ChineseStandard.us -- Field Test Asia Pte. Ltd.
YS/T 820.3-2012 English PDF (YS/T820.3-2012)
YS/T 820.3-2012 English PDF (YS/T820.3-2012)
Regular price
$120.00
Regular price
Sale price
$120.00
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
YS/T 820.3-2012: Methods for chemical analysis of laterite nickel ores - Part 3: Determination of total iron content - Potassium dichromate titration
Delivery: 9 seconds. Download (& Email) true-PDF + Invoice.
Get Quotation: Click YS/T 820.3-2012 (Self-service in 1-minute)
Historical versions (Master-website): YS/T 820.3-2012
Preview True-PDF (Reload/Scroll-down if blank)
YS/T 820.3-2012
YS
NONFERROUS METALLURGY INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 73.060
D 04
Methods for chemical analysis of laterite nickel ores -
Part 3. Determination of total iron content -
Potassium dichromate titration
ISSUED ON. NOVEMBER 07, 2012
IMPLEMENTED ON. MARCH 01, 2013
Issued by. Ministry of Industry and Information Technology of the
People's Republic of China.
Table of Contents
Foreword ... 3
1 Scope ... 6
2 Normative references ... 6
3 Method summary ... 6
4 Reagents ... 6
5 Specimen ... 8
6 Analysis steps ... 8
7 Calculation of analysis results ... 9
8 Precision ... 10
9 Test report ... 10
Foreword
This Part was drafted in accordance with the rules given in GB/T 1.1-2009.
YS/T 820-2012 “Methods for chemical analysis of laterite nickel ores” consists
of 26 parts.
- Part 1. Determination of nickel content - Flame atomic absorption
spectrometry;
- Part 2. Determination of nickel content - Dimethylglyoxime
spectrophotometry;
- Part 3. Determination of total iron content - Potassium dichromate titration;
- Part 4. Determination of phosphorus content - Phosphorus molybdenum
blue spectrophotometry;
- Part 5. Determination of cobalt content - Flame atomic absorption
spectrometry;
- Part 6. Determination of copper content - Flame atomic absorption
spectrometry;
- Part 7. Determination of calcium and magnesium content – Flame atomic
absorption spectrometry;
- Part 8. Determination of silica content - Potassium silicafluoride titrimetric
method;
- Part 9. Determination of scandium and cadmium contents - Inductively
coupled plasma mass spectrometry;
- Part 10. Determination of calcium, cobalt, copper, magnesium, manganese,
nickel, phosphate and zinc content - Inductively coupled plasma atomic
emission spectrometry;
- Part 11. Determination of fluorine and chlorine contents - Ion
chromatography;
- Part 12. Determination of manganese content - Flame atomic absorption
spectrometry;
- Part 13. Determination of lead content - Flame atomic absorption
spectrometry;
- Part 14. Determination of the zinc content - Flame atomic absorption
spectrometry;
Methods for chemical analysis of laterite nickel ores -
Part 3. Determination of total iron content -
Potassium dichromate titration
1 Scope
This Part of YS/T 820 specifies the determination method of total iron content
in laterite nickel ores.
This Part is applicable to the determination of total iron content in laterite nickel
ores. The determination range is 7.00%~55.00%.
2 Normative references
The following referenced documents are indispensable for the application of
this document. For dated references, only the edition cited applies. For undated
references, the latest edition of the referenced document (including any
amendments) applies.
YS/T 820.24-2012, Methods for chemical analysis of laterite nickel ores -
Part 24. Determination hygroscopic moisture content - Gravimetric method
3 Method summary
The test material is melted with sodium peroxide. Use water to leach the cooled
melt out. Then use hydrochloric acid to acidize. Most of the iron is reduced by
stannous chloride. The remaining iron is reduced by titanium trichloride. Use
potassium dichromate solution to oxidize the excess reducing agent. Use
sodium diphenylamine sulfonate as indicator. Use potassium dichromate
standard titration solution to titrate the reduced amount of iron.
4 Reagents
Unless otherwise stated, only analytically pure reagents and distilled or
deionized water or water with equivalent purity are used in the analysis.
4.1 Sodium peroxide.
4.2 Hydrochloric acid (ρ1.19 g/mL).
5 Specimen
5.1 Specimen
The granularity of the specimen shall be less than 160 µm.
5.2 Determination of wet reserved water amount
While analyzing specimen, determine the wet reserved water amount according
to YS/T 820.24-2012.
6 Analysis steps
6.1 Test material
Weigh 0.20 g of specimen, to the nearest of 0. 0001 g.
6.2 Determination frequency
Carry out the determination twice, independently. Take the mean value.
6.3 Blank test
Carry out the blank test with test material (see 6.4.4 for requirements).
6.4 Determination
6.4.1 Decomposition of test material
Place the test material (6.1) in a 30mL corundum crucible. Add 2g~3g of sodium
peroxide (4.1). Mix well. Then add 1 g of sodium peroxide (4.1) to cover. Melt
in a muffle furnace at 800°C for 8min~10min. Take out and cool for a while.
Leach in a 300mL beaker that containing 40mL of water. Add 25 mL of
hydrochloric acid (4.2). Heat on an electric furnace to near boiling.
6.4.2 Reduction
Immediately drop stannous chloride solution (4.9), reduced iron (III). Keep
stirring the crucible and solution till the solution becomes light yellow. Clean the
inner wall of the beaker with small amount of water. Cool with running water to
room temperature. Add 15 drops of sodium tungstate solution (4.13) as indicator.
Reduce the remaining iron (III) with titanium trichloride solution. Keep stirring
till the solution becomes blue.
6.4.3 Titration
While stirring, drop the potassium dichromate solution (4.8) till colorless. Add
8 Precision
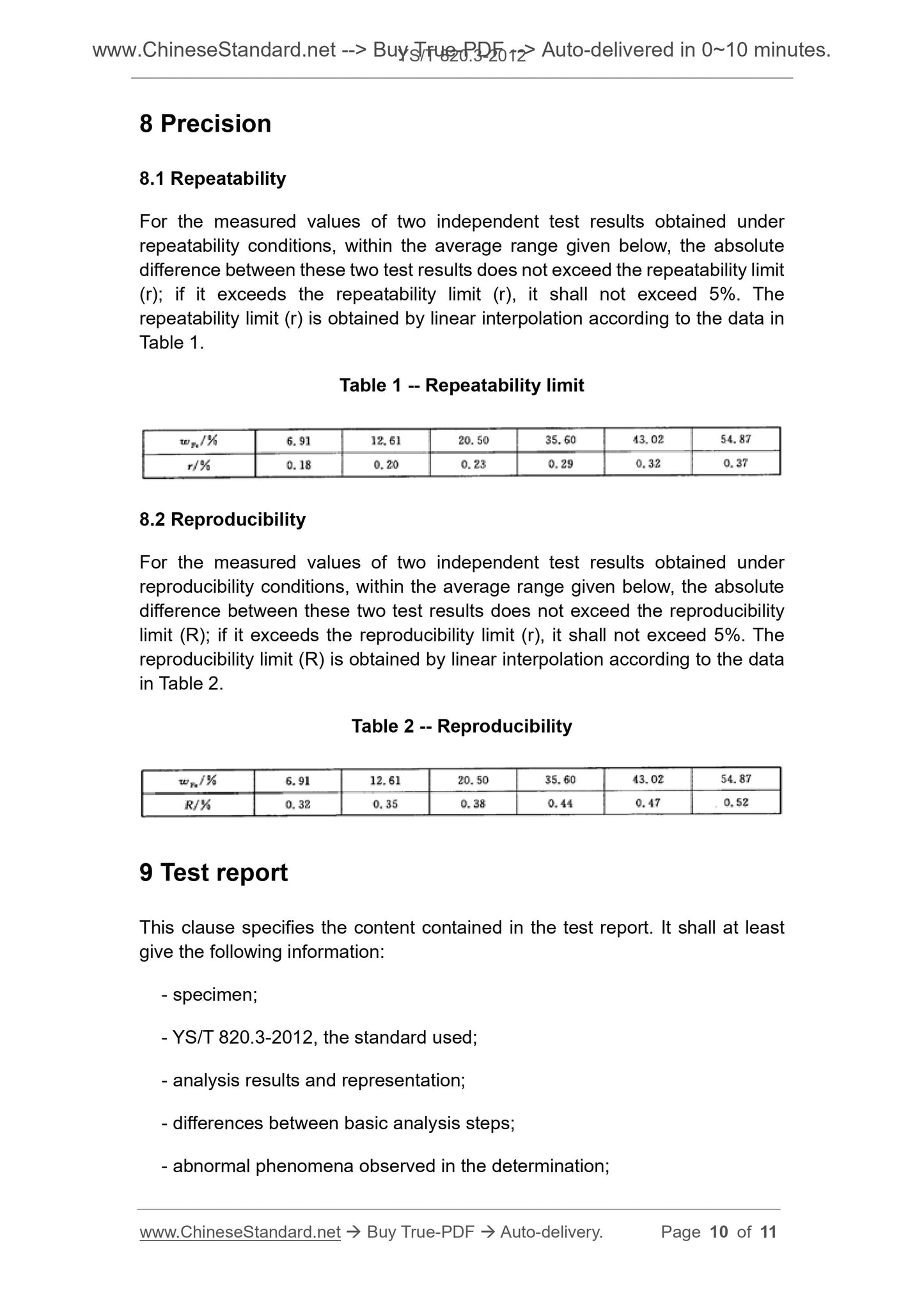
8.1 Repeatability
For the measured values of two independent test results obtained under
repeatability conditions, within the average range given below, the absolute
difference between these two test results does not exceed the repeatability limit
(r); if it exceeds the repeatability limit (r), it shall not exceed 5%. The
repeatability limit (r) is obtained by linear interpolation according to the data in
Table 1.
Table 1 -- Repeatability limit
8.2 Reproducibility
For the measured values of two independent test results obtained under
reproducibility conditions, within the average range given below, the absolute
difference between these two test results does not exceed the reproducibility
limit (R); if it exceeds the reproducibility limit (r), it shall not exceed 5%. The
reproducibility limit (R) is obtained by linear interpolation according to the data
in Table 2.
Table 2 -- Reproducibility
9 Test report
This clause specifies the content contained in the test report. It shall at least
give the following information.
- specimen;
- YS/T 820.3-2012, the standard used;
- analysis results and representation;
- differences between basic analysis steps;
- abnormal phenomena observed in the determination;
YS/T 820.3-2012
YS
NONFERROUS METALLURGY INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 73.060
D 04
Methods for chemical analysis of laterite nickel ores -
Part 3. Determination of total iron content -
Potassium dichromate titration
ISSUED ON. NOVEMBER 07, 2012
IMPLEMENTED ON. MARCH 01, 2013
Issued by. Ministry of Industry and Information Technology of the
People's Republic of China.
Table of Contents
Foreword ... 3
1 Scope ... 6
2 Normative references ... 6
3 Method summary ... 6
4 Reagents ... 6
5 Specimen ... 8
6 Analysis steps ... 8
7 Calculation of analysis results ... 9
8 Precision ... 10
9 Test report ... 10
Foreword
This Part was drafted in accordance with the rules given in GB/T 1.1-2009.
YS/T 820-2012 “Methods for chemical analysis of laterite nickel ores” consists
of 26 parts.
- Part 1. Determination of nickel content - Flame atomic absorption
spectrometry;
- Part 2. Determination of nickel content - Dimethylglyoxime
spectrophotometry;
- Part 3. Determination of total iron content - Potassium dichromate titration;
- Part 4. Determination of phosphorus content - Phosphorus molybdenum
blue spectrophotometry;
- Part 5. Determination of cobalt content - Flame atomic absorption
spectrometry;
- Part 6. Determination of copper content - Flame atomic absorption
spectrometry;
- Part 7. Determination of calcium and magnesium content – Flame atomic
absorption spectrometry;
- Part 8. Determination of silica content - Potassium silicafluoride titrimetric
method;
- Part 9. Determination of scandium and cadmium contents - Inductively
coupled plasma mass spectrometry;
- Part 10. Determination of calcium, cobalt, copper, magnesium, manganese,
nickel, phosphate and zinc content - Inductively coupled plasma atomic
emission spectrometry;
- Part 11. Determination of fluorine and chlorine contents - Ion
chromatography;
- Part 12. Determination of manganese content - Flame atomic absorption
spectrometry;
- Part 13. Determination of lead content - Flame atomic absorption
spectrometry;
- Part 14. Determination of the zinc content - Flame atomic absorption
spectrometry;
Methods for chemical analysis of laterite nickel ores -
Part 3. Determination of total iron content -
Potassium dichromate titration
1 Scope
This Part of YS/T 820 specifies the determination method of total iron content
in laterite nickel ores.
This Part is applicable to the determination of total iron content in laterite nickel
ores. The determination range is 7.00%~55.00%.
2 Normative references
The following referenced documents are indispensable for the application of
this document. For dated references, only the edition cited applies. For undated
references, the latest edition of the referenced document (including any
amendments) applies.
YS/T 820.24-2012, Methods for chemical analysis of laterite nickel ores -
Part 24. Determination hygroscopic moisture content - Gravimetric method
3 Method summary
The test material is melted with sodium peroxide. Use water to leach the cooled
melt out. Then use hydrochloric acid to acidize. Most of the iron is reduced by
stannous chloride. The remaining iron is reduced by titanium trichloride. Use
potassium dichromate solution to oxidize the excess reducing agent. Use
sodium diphenylamine sulfonate as indicator. Use potassium dichromate
standard titration solution to titrate the reduced amount of iron.
4 Reagents
Unless otherwise stated, only analytically pure reagents and distilled or
deionized water or water with equivalent purity are used in the analysis.
4.1 Sodium peroxide.
4.2 Hydrochloric acid (ρ1.19 g/mL).
5 Specimen
5.1 Specimen
The granularity of the specimen shall be less than 160 µm.
5.2 Determination of wet reserved water amount
While analyzing specimen, determine the wet reserved water amount according
to YS/T 820.24-2012.
6 Analysis steps
6.1 Test material
Weigh 0.20 g of specimen, to the nearest of 0. 0001 g.
6.2 Determination frequency
Carry out the determination twice, independently. Take the mean value.
6.3 Blank test
Carry out the blank test with test material (see 6.4.4 for requirements).
6.4 Determination
6.4.1 Decomposition of test material
Place the test material (6.1) in a 30mL corundum crucible. Add 2g~3g of sodium
peroxide (4.1). Mix well. Then add 1 g of sodium peroxide (4.1) to cover. Melt
in a muffle furnace at 800°C for 8min~10min. Take out and cool for a while.
Leach in a 300mL beaker that containing 40mL of water. Add 25 mL of
hydrochloric acid (4.2). Heat on an electric furnace to near boiling.
6.4.2 Reduction
Immediately drop stannous chloride solution (4.9), reduced iron (III). Keep
stirring the crucible and solution till the solution becomes light yellow. Clean the
inner wall of the beaker with small amount of water. Cool with running water to
room temperature. Add 15 drops of sodium tungstate solution (4.13) as indicator.
Reduce the remaining iron (III) with titanium trichloride solution. Keep stirring
till the solution becomes blue.
6.4.3 Titration
While stirring, drop the potassium dichromate solution (4.8) till colorless. Add
8 Precision
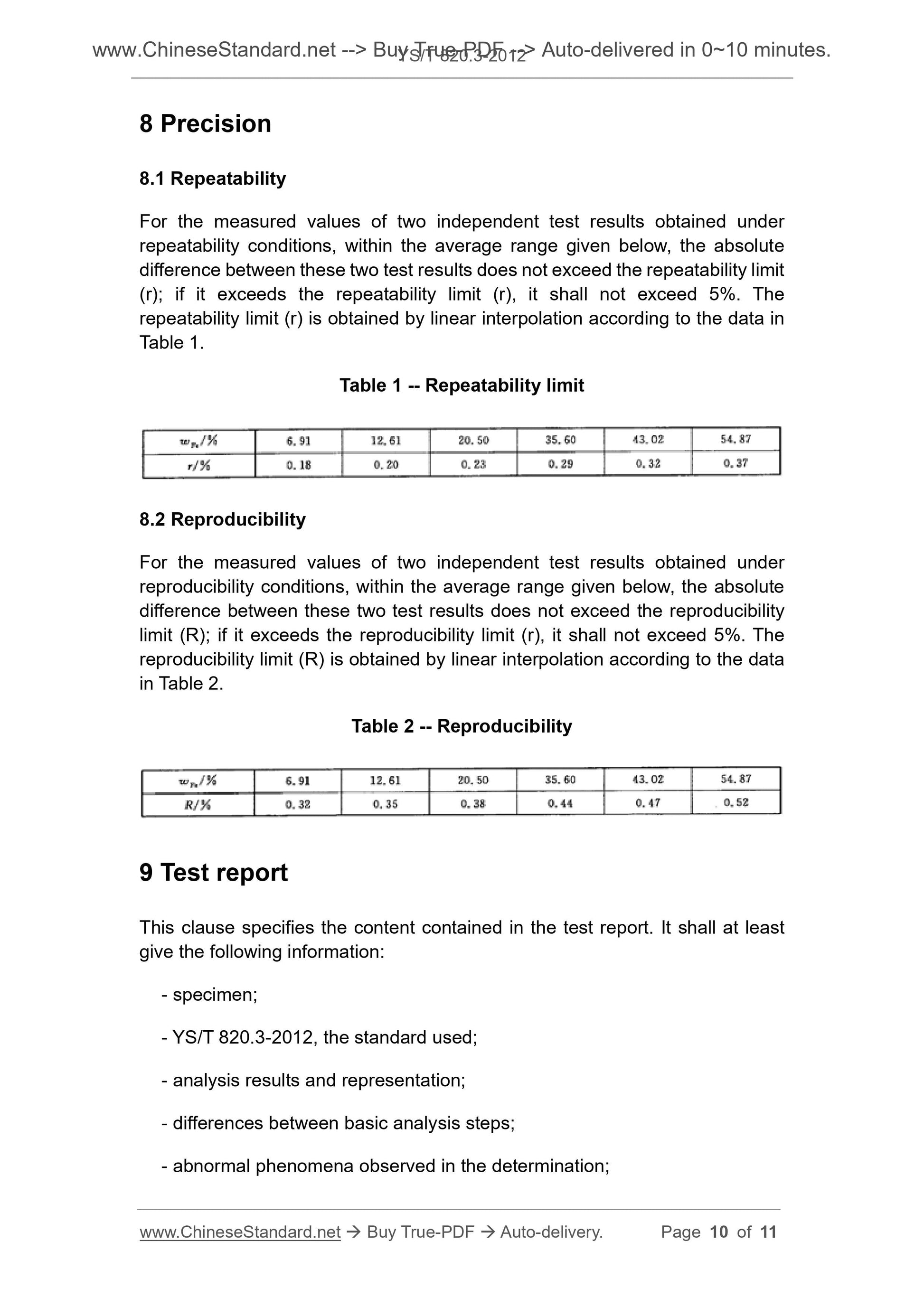
8.1 Repeatability
For the measured values of two independent test results obtained under
repeatability conditions, within the average range given below, the absolute
difference between these two test results does not exceed the repeatability limit
(r); if it exceeds the repeatability limit (r), it shall not exceed 5%. The
repeatability limit (r) is obtained by linear interpolation according to the data in
Table 1.
Table 1 -- Repeatability limit
8.2 Reproducibility
For the measured values of two independent test results obtained under
reproducibility conditions, within the average range given below, the absolute
difference between these two test results does not exceed the reproducibility
limit (R); if it exceeds the reproducibility limit (r), it shall not exceed 5%. The
reproducibility limit (R) is obtained by linear interpolation according to the data
in Table 2.
Table 2 -- Reproducibility
9 Test report
This clause specifies the content contained in the test report. It shall at least
give the following information.
- specimen;
- YS/T 820.3-2012, the standard used;
- analysis results and representation;
- differences between basic analysis steps;
- abnormal phenomena observed in the determination;
Delivery: 9 seconds. Download (& Email) true-PDF + Invoice.
Get Quotation: Click YS/T 820.3-2012 (Self-service in 1-minute)
Historical versions (Master-website): YS/T 820.3-2012
Preview True-PDF (Reload/Scroll-down if blank)
YS/T 820.3-2012
YS
NONFERROUS METALLURGY INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 73.060
D 04
Methods for chemical analysis of laterite nickel ores -
Part 3. Determination of total iron content -
Potassium dichromate titration
ISSUED ON. NOVEMBER 07, 2012
IMPLEMENTED ON. MARCH 01, 2013
Issued by. Ministry of Industry and Information Technology of the
People's Republic of China.
Table of Contents
Foreword ... 3
1 Scope ... 6
2 Normative references ... 6
3 Method summary ... 6
4 Reagents ... 6
5 Specimen ... 8
6 Analysis steps ... 8
7 Calculation of analysis results ... 9
8 Precision ... 10
9 Test report ... 10
Foreword
This Part was drafted in accordance with the rules given in GB/T 1.1-2009.
YS/T 820-2012 “Methods for chemical analysis of laterite nickel ores” consists
of 26 parts.
- Part 1. Determination of nickel content - Flame atomic absorption
spectrometry;
- Part 2. Determination of nickel content - Dimethylglyoxime
spectrophotometry;
- Part 3. Determination of total iron content - Potassium dichromate titration;
- Part 4. Determination of phosphorus content - Phosphorus molybdenum
blue spectrophotometry;
- Part 5. Determination of cobalt content - Flame atomic absorption
spectrometry;
- Part 6. Determination of copper content - Flame atomic absorption
spectrometry;
- Part 7. Determination of calcium and magnesium content – Flame atomic
absorption spectrometry;
- Part 8. Determination of silica content - Potassium silicafluoride titrimetric
method;
- Part 9. Determination of scandium and cadmium contents - Inductively
coupled plasma mass spectrometry;
- Part 10. Determination of calcium, cobalt, copper, magnesium, manganese,
nickel, phosphate and zinc content - Inductively coupled plasma atomic
emission spectrometry;
- Part 11. Determination of fluorine and chlorine contents - Ion
chromatography;
- Part 12. Determination of manganese content - Flame atomic absorption
spectrometry;
- Part 13. Determination of lead content - Flame atomic absorption
spectrometry;
- Part 14. Determination of the zinc content - Flame atomic absorption
spectrometry;
Methods for chemical analysis of laterite nickel ores -
Part 3. Determination of total iron content -
Potassium dichromate titration
1 Scope
This Part of YS/T 820 specifies the determination method of total iron content
in laterite nickel ores.
This Part is applicable to the determination of total iron content in laterite nickel
ores. The determination range is 7.00%~55.00%.
2 Normative references
The following referenced documents are indispensable for the application of
this document. For dated references, only the edition cited applies. For undated
references, the latest edition of the referenced document (including any
amendments) applies.
YS/T 820.24-2012, Methods for chemical analysis of laterite nickel ores -
Part 24. Determination hygroscopic moisture content - Gravimetric method
3 Method summary
The test material is melted with sodium peroxide. Use water to leach the cooled
melt out. Then use hydrochloric acid to acidize. Most of the iron is reduced by
stannous chloride. The remaining iron is reduced by titanium trichloride. Use
potassium dichromate solution to oxidize the excess reducing agent. Use
sodium diphenylamine sulfonate as indicator. Use potassium dichromate
standard titration solution to titrate the reduced amount of iron.
4 Reagents
Unless otherwise stated, only analytically pure reagents and distilled or
deionized water or water with equivalent purity are used in the analysis.
4.1 Sodium peroxide.
4.2 Hydrochloric acid (ρ1.19 g/mL).
5 Specimen
5.1 Specimen
The granularity of the specimen shall be less than 160 µm.
5.2 Determination of wet reserved water amount
While analyzing specimen, determine the wet reserved water amount according
to YS/T 820.24-2012.
6 Analysis steps
6.1 Test material
Weigh 0.20 g of specimen, to the nearest of 0. 0001 g.
6.2 Determination frequency
Carry out the determination twice, independently. Take the mean value.
6.3 Blank test
Carry out the blank test with test material (see 6.4.4 for requirements).
6.4 Determination
6.4.1 Decomposition of test material
Place the test material (6.1) in a 30mL corundum crucible. Add 2g~3g of sodium
peroxide (4.1). Mix well. Then add 1 g of sodium peroxide (4.1) to cover. Melt
in a muffle furnace at 800°C for 8min~10min. Take out and cool for a while.
Leach in a 300mL beaker that containing 40mL of water. Add 25 mL of
hydrochloric acid (4.2). Heat on an electric furnace to near boiling.
6.4.2 Reduction
Immediately drop stannous chloride solution (4.9), reduced iron (III). Keep
stirring the crucible and solution till the solution becomes light yellow. Clean the
inner wall of the beaker with small amount of water. Cool with running water to
room temperature. Add 15 drops of sodium tungstate solution (4.13) as indicator.
Reduce the remaining iron (III) with titanium trichloride solution. Keep stirring
till the solution becomes blue.
6.4.3 Titration
While stirring, drop the potassium dichromate solution (4.8) till colorless. Add
8 Precision
8.1 Repeatability
For the measured values of two independent test results obtained under
repeatability conditions, within the average range given below, the absolute
difference between these two test results does not exceed the repeatability limit
(r); if it exceeds the repeatability limit (r), it shall not exceed 5%. The
repeatability limit (r) is obtained by linear interpolation according to the data in
Table 1.
Table 1 -- Repeatability limit
8.2 Reproducibility
For the measured values of two independent test results obtained under
reproducibility conditions, within the average range given below, the absolute
difference between these two test results does not exceed the reproducibility
limit (R); if it exceeds the reproducibility limit (r), it shall not exceed 5%. The
reproducibility limit (R) is obtained by linear interpolation according to the data
in Table 2.
Table 2 -- Reproducibility
9 Test report
This clause specifies the content contained in the test report. It shall at least
give the following information.
- specimen;
- YS/T 820.3-2012, the standard used;
- analysis results and representation;
- differences between basic analysis steps;
- abnormal phenomena observed in the determination;
YS/T 820.3-2012
YS
NONFERROUS METALLURGY INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 73.060
D 04
Methods for chemical analysis of laterite nickel ores -
Part 3. Determination of total iron content -
Potassium dichromate titration
ISSUED ON. NOVEMBER 07, 2012
IMPLEMENTED ON. MARCH 01, 2013
Issued by. Ministry of Industry and Information Technology of the
People's Republic of China.
Table of Contents
Foreword ... 3
1 Scope ... 6
2 Normative references ... 6
3 Method summary ... 6
4 Reagents ... 6
5 Specimen ... 8
6 Analysis steps ... 8
7 Calculation of analysis results ... 9
8 Precision ... 10
9 Test report ... 10
Foreword
This Part was drafted in accordance with the rules given in GB/T 1.1-2009.
YS/T 820-2012 “Methods for chemical analysis of laterite nickel ores” consists
of 26 parts.
- Part 1. Determination of nickel content - Flame atomic absorption
spectrometry;
- Part 2. Determination of nickel content - Dimethylglyoxime
spectrophotometry;
- Part 3. Determination of total iron content - Potassium dichromate titration;
- Part 4. Determination of phosphorus content - Phosphorus molybdenum
blue spectrophotometry;
- Part 5. Determination of cobalt content - Flame atomic absorption
spectrometry;
- Part 6. Determination of copper content - Flame atomic absorption
spectrometry;
- Part 7. Determination of calcium and magnesium content – Flame atomic
absorption spectrometry;
- Part 8. Determination of silica content - Potassium silicafluoride titrimetric
method;
- Part 9. Determination of scandium and cadmium contents - Inductively
coupled plasma mass spectrometry;
- Part 10. Determination of calcium, cobalt, copper, magnesium, manganese,
nickel, phosphate and zinc content - Inductively coupled plasma atomic
emission spectrometry;
- Part 11. Determination of fluorine and chlorine contents - Ion
chromatography;
- Part 12. Determination of manganese content - Flame atomic absorption
spectrometry;
- Part 13. Determination of lead content - Flame atomic absorption
spectrometry;
- Part 14. Determination of the zinc content - Flame atomic absorption
spectrometry;
Methods for chemical analysis of laterite nickel ores -
Part 3. Determination of total iron content -
Potassium dichromate titration
1 Scope
This Part of YS/T 820 specifies the determination method of total iron content
in laterite nickel ores.
This Part is applicable to the determination of total iron content in laterite nickel
ores. The determination range is 7.00%~55.00%.
2 Normative references
The following referenced documents are indispensable for the application of
this document. For dated references, only the edition cited applies. For undated
references, the latest edition of the referenced document (including any
amendments) applies.
YS/T 820.24-2012, Methods for chemical analysis of laterite nickel ores -
Part 24. Determination hygroscopic moisture content - Gravimetric method
3 Method summary
The test material is melted with sodium peroxide. Use water to leach the cooled
melt out. Then use hydrochloric acid to acidize. Most of the iron is reduced by
stannous chloride. The remaining iron is reduced by titanium trichloride. Use
potassium dichromate solution to oxidize the excess reducing agent. Use
sodium diphenylamine sulfonate as indicator. Use potassium dichromate
standard titration solution to titrate the reduced amount of iron.
4 Reagents
Unless otherwise stated, only analytically pure reagents and distilled or
deionized water or water with equivalent purity are used in the analysis.
4.1 Sodium peroxide.
4.2 Hydrochloric acid (ρ1.19 g/mL).
5 Specimen
5.1 Specimen
The granularity of the specimen shall be less than 160 µm.
5.2 Determination of wet reserved water amount
While analyzing specimen, determine the wet reserved water amount according
to YS/T 820.24-2012.
6 Analysis steps
6.1 Test material
Weigh 0.20 g of specimen, to the nearest of 0. 0001 g.
6.2 Determination frequency
Carry out the determination twice, independently. Take the mean value.
6.3 Blank test
Carry out the blank test with test material (see 6.4.4 for requirements).
6.4 Determination
6.4.1 Decomposition of test material
Place the test material (6.1) in a 30mL corundum crucible. Add 2g~3g of sodium
peroxide (4.1). Mix well. Then add 1 g of sodium peroxide (4.1) to cover. Melt
in a muffle furnace at 800°C for 8min~10min. Take out and cool for a while.
Leach in a 300mL beaker that containing 40mL of water. Add 25 mL of
hydrochloric acid (4.2). Heat on an electric furnace to near boiling.
6.4.2 Reduction
Immediately drop stannous chloride solution (4.9), reduced iron (III). Keep
stirring the crucible and solution till the solution becomes light yellow. Clean the
inner wall of the beaker with small amount of water. Cool with running water to
room temperature. Add 15 drops of sodium tungstate solution (4.13) as indicator.
Reduce the remaining iron (III) with titanium trichloride solution. Keep stirring
till the solution becomes blue.
6.4.3 Titration
While stirring, drop the potassium dichromate solution (4.8) till colorless. Add
8 Precision
8.1 Repeatability
For the measured values of two independent test results obtained under
repeatability conditions, within the average range given below, the absolute
difference between these two test results does not exceed the repeatability limit
(r); if it exceeds the repeatability limit (r), it shall not exceed 5%. The
repeatability limit (r) is obtained by linear interpolation according to the data in
Table 1.
Table 1 -- Repeatability limit
8.2 Reproducibility
For the measured values of two independent test results obtained under
reproducibility conditions, within the average range given below, the absolute
difference between these two test results does not exceed the reproducibility
limit (R); if it exceeds the reproducibility limit (r), it shall not exceed 5%. The
reproducibility limit (R) is obtained by linear interpolation according to the data
in Table 2.
Table 2 -- Reproducibility
9 Test report
This clause specifies the content contained in the test report. It shall at least
give the following information.
- specimen;
- YS/T 820.3-2012, the standard used;
- analysis results and representation;
- differences between basic analysis steps;
- abnormal phenomena observed in the determination;
Share