1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY 0068.3-2008 English PDF (YY0068.3-2008)
YY 0068.3-2008 English PDF (YY0068.3-2008)
Regular price
$145.00 USD
Regular price
Sale price
$145.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY 0068.3-2008
Historical versions: YY 0068.3-2008
Preview True-PDF (Reload/Scroll if blank)
YY 0068.3-2008: Medical endoscopes. Rigid endoscope. Part 3: Marking and instruction manual
YY 0068.3-2008
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040
C 40
Medical endoscopes – Rigid endoscope –
Part 3. Marking and instruction manual
ISSUED ON. OCTOBER 17, 2008
IMPLEMENTED ON. JUNE 01, 2010
Issued by. China Food and Drug Administration
3. No action is required - Full-copy of this standard will be automatically and
immediately delivered to your EMAIL address in 0~60 minutes.
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative references ... 4
3 Requirements ... 4
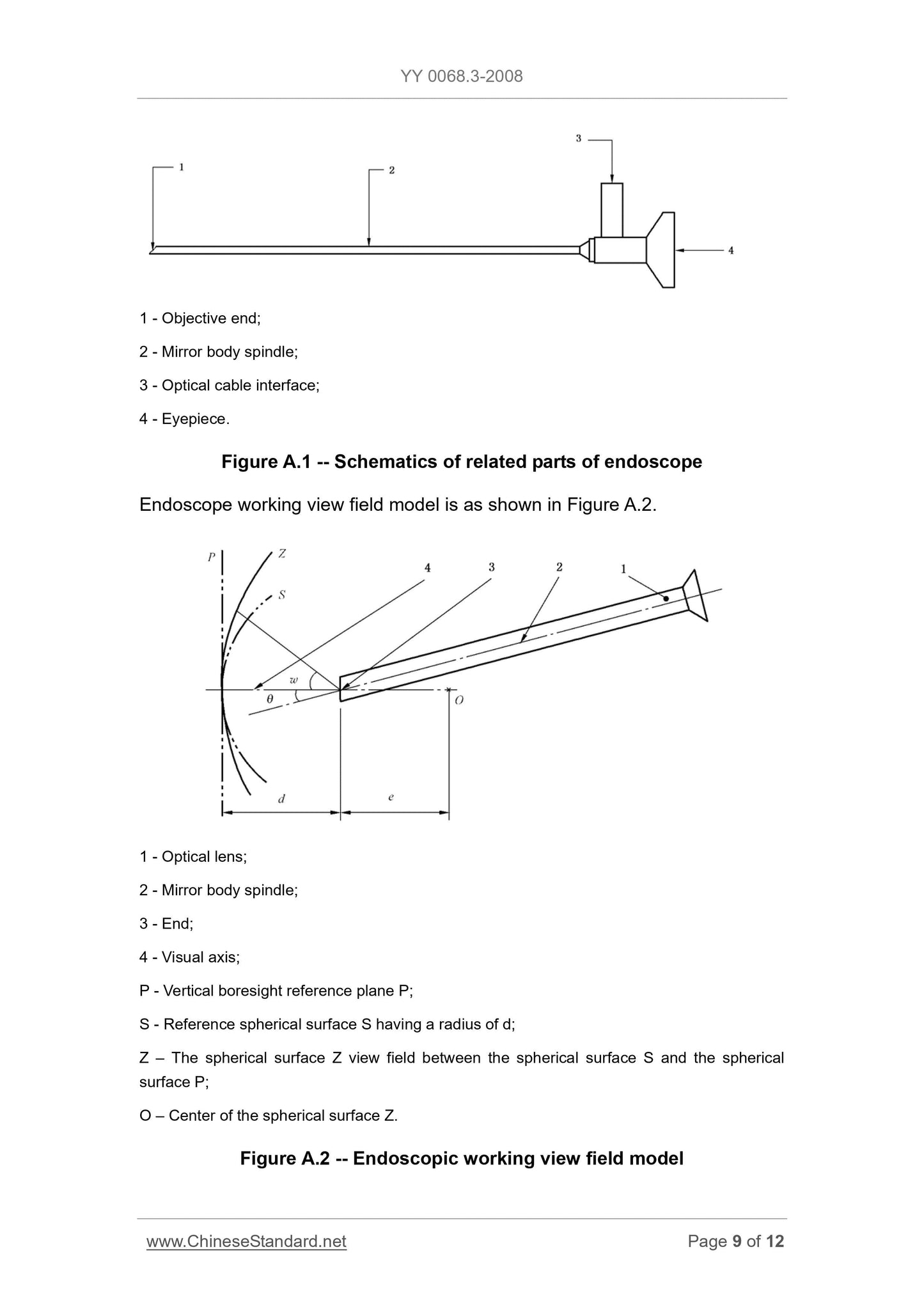
Appendix A (Informative) Examples of instruction manual ... 7
Foreword
YY 0068 “Medical endoscopes - Rigid endoscope” is divided into four parts.
- Part 1. Optical properties and test methods;
- Part 2. Mechanical properties and test methods
- Part 3. Marking and instruction manual;
- Part 4. Fundamental requirement.
This part is part 3 of YY 0068.
This part corresponds to the ISO 8600-1.2005 “Optics and photonics - Medical
endoscopes and endotherapy devices - Part 1. General requirements”, AND its
consistency with ISO 8600-1. 2005 is non-equivalent.
Appendix A of this part is an informative appendix.
This part was approved by the China Food and Drug Administration.
This part was proposed by AND shall be under the jurisdiction of the National
Optical and Optical Instrument Standardization Technical Committee Medical
Optical and Instrument Technical Committee (SAC/TC 103 /SC 1).
The drafting organizations of this part. China Food and Drug Administration
Hangzhou Medical Device Quality Supervision and Inspection Center.
The main drafters of this part. Yan Qinglai, Mao Xinxin, Jia Xiaohang, He Tao,
Qi Weiming.
Medical endoscopes – Rigid endoscope –
Part 3. Marking and instruction manual
1 Scope
This part of YY 0068 specifies the requirements for the marking and instruction
manual of rigid endoscope.
2 Normative references
The provisions in following documents become the provisions of this part
through reference in this part of YY 0068. For the dated references, the
subsequent amendments (excluding corrections) or revisions do not apply to
this part; however, parties who reach an agreement based on this part are
encouraged to study if the latest versions of these documents are applicable.
For undated references, the latest edition of the referenced document applies.
GB 9706.19 Medical electrical equipment - Part 2. Particular requirements
for the safety of endoscopic equipment (GB 9706.19-2000, idt IEC
60601-2-18.1996)
YY 0068.1 Medical Endoscopes - Rigid Endoscope - Part 1. Optical
properties and test methods
YY 0068.2 Medical endoscopes - Rigid endoscope - Part 2. Mechanical
properties and test methods
ISO 10526.1999 CIE S 005 CIE Standard illuminants for colorimetry
3 Requirements
3.1 Marking
3.1.1 Minimum marking
Each endoscope shall have at least the following markings.
a) An identification number and/or other markings sufficient to identify the
endoscope and manufacturer;
Appendix A
(Informative)
Examples of instruction manual
Instruction manual
A.1 Please read the entire contents of this manual thoroughly before using the
XXX endoscope manufactured by the XXX Endoscope Co., Ltd. Failure to
follow the instructions and warnings in this manual can have serious
consequences for the patient.
A.2 Endoscopes shall only be used by practicing physicians and trained
medical professionals.
A.3 This endoscope is used for XXX surgery and diagnosis and treatment in
the surgery.
A.4 The endoscope can be used in conjunction with the video and light source
equipment to observe the body cavity situation.
Correct use method. First MAKE sure that the endoscope is properly cleaned,
disinfected and/or sterilized before use (refer to the cleaning, sterilizing and
disinfection parts for detailed procedures); CHECK the endoscope for damage
and normal function; FOLLOW all the manufacturer's instructions to operate all
video and light source equipment; CONNECT the fiber optic cable to the fiber
optic cable interface on the endoscope; FOLLOW the appropriate medical
surgical code, and REFER to the appropriate surgical literatures for the
endoscopic surgical entry points.
A.5 Endoscope identification and parameters, including the contents in Table A.
1.
Table A.1 -- Identification and parameters
No. Items
Manufacturer's name. xx endoscope Co., Ltd.
Manufacturer’s address. No. XXX XXX road, XXX economic development zone, XXX city, XXX
province
2 Name. xx endoscope Identification number. XXXXXX
3 Insertion part maximum width. 4 mm (round) Working length. 200 mm
4 The minimum width of the instrument channel. /
A.7 Applicable instructions. The endoscope can be used in conjunction with
various camera systems, cold light sources, high-frequency generators, and
high-frequency equipment which comply with GB 9706.19.
WARNING. The minimum required light intensity for the light source to achieve
the best endoscopic field of view illumination is always adjusted, either for
direct or video use. The higher the light intensity of the light source, the greater
the amount of heat generated at the end of the endoscope.
A.8 The inspection instructions on the endoscope to be reasonably
guaranteed by following the operating procedures.
Check the products. Visual inspection of the product to ensure that it is no rust,
no indentation, and no scratches.
Check the image quality. through the optical tube, it shall be able to clearly
view the text about 30 mm away from the end of the objective lens.
A.9 Cleaning instructions on the reusable endoscope
A.9.1 After use, it shall be thoroughly cleaned by flowing water to remove the
residual substances such as blood and mucus, AND wipe it dry.
A.9.2 After drying the endoscope, it is placed in the multi-enzyme washing
solution for soaking.
A.9.3 Thoroughly CLEAN the endoscopic components, the tube inside shall be
thoroughly washed with a high-pressure water-gun, the removable parts must
be disassembled and cleaned, AND cleaned with an ultrasonic cleaner for 5
min ~ 10 min.
A.9.4 USE a soft brush to thoroughly brush the tube inside, AND pay attention
to avoid scratching the lens surface.
A.9.5 Instructions for special cleaning tools or equipment
It can only use the ultrasonic cleaner that the manufacturer of the cleaning
machine manufactures to clean the endoscope for cleaning the endoscope.
For the cleaning process, REFER to the manual of the ultrasonic cleaner.
A.10 Instruction of endoscope’s bearable special disinfection and sterilization
environment
A.10.1 Disinfection instructions
Endoscopes can be chemically sterilized using advanced disinfection solutions
containing 2% glutaraldehyde specifically for endoscope. It shall avoid using
Get QUOTATION in 1-minute: Click YY 0068.3-2008
Historical versions: YY 0068.3-2008
Preview True-PDF (Reload/Scroll if blank)
YY 0068.3-2008: Medical endoscopes. Rigid endoscope. Part 3: Marking and instruction manual
YY 0068.3-2008
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040
C 40
Medical endoscopes – Rigid endoscope –
Part 3. Marking and instruction manual
ISSUED ON. OCTOBER 17, 2008
IMPLEMENTED ON. JUNE 01, 2010
Issued by. China Food and Drug Administration
3. No action is required - Full-copy of this standard will be automatically and
immediately delivered to your EMAIL address in 0~60 minutes.
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative references ... 4
3 Requirements ... 4
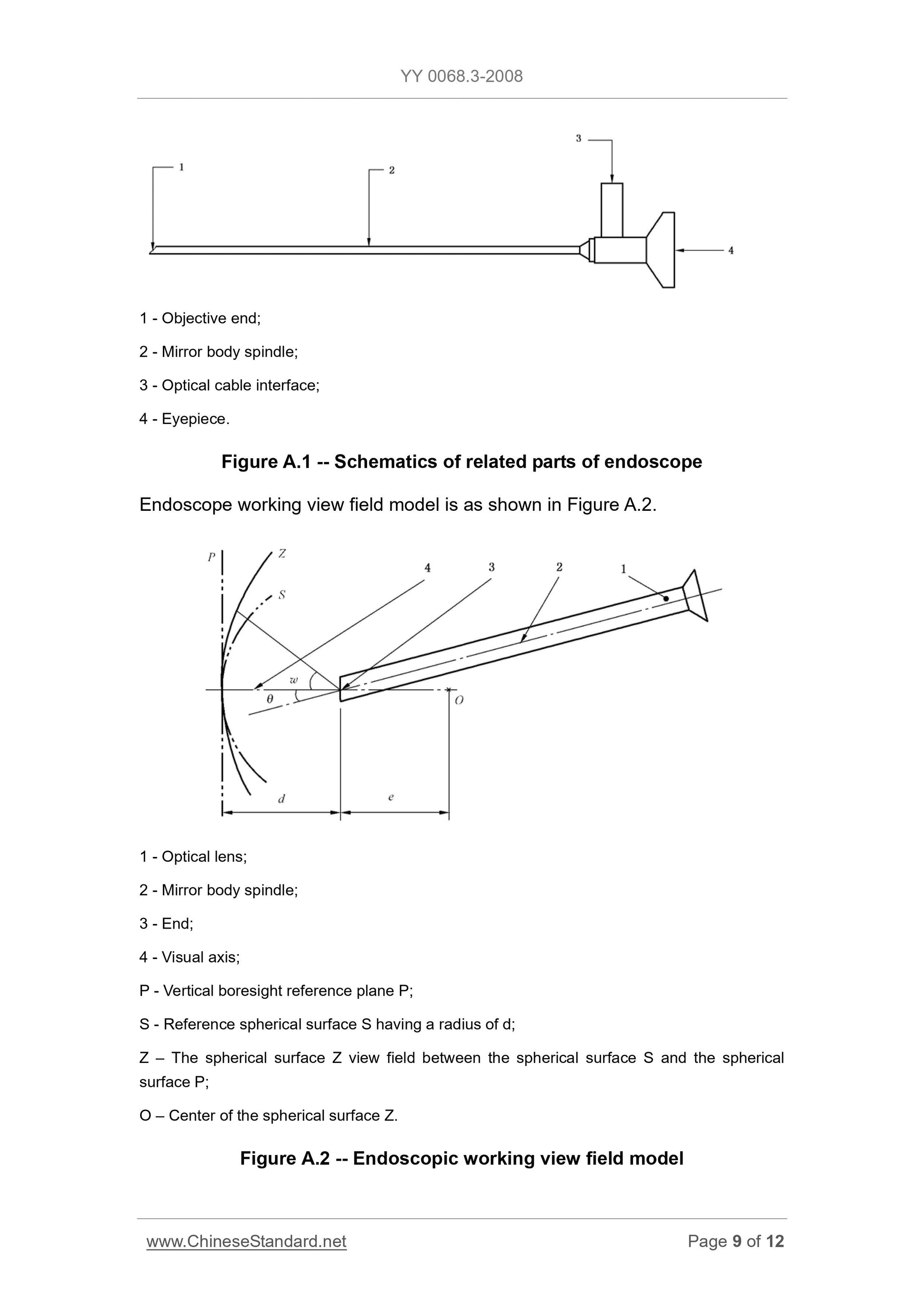
Appendix A (Informative) Examples of instruction manual ... 7
Foreword
YY 0068 “Medical endoscopes - Rigid endoscope” is divided into four parts.
- Part 1. Optical properties and test methods;
- Part 2. Mechanical properties and test methods
- Part 3. Marking and instruction manual;
- Part 4. Fundamental requirement.
This part is part 3 of YY 0068.
This part corresponds to the ISO 8600-1.2005 “Optics and photonics - Medical
endoscopes and endotherapy devices - Part 1. General requirements”, AND its
consistency with ISO 8600-1. 2005 is non-equivalent.
Appendix A of this part is an informative appendix.
This part was approved by the China Food and Drug Administration.
This part was proposed by AND shall be under the jurisdiction of the National
Optical and Optical Instrument Standardization Technical Committee Medical
Optical and Instrument Technical Committee (SAC/TC 103 /SC 1).
The drafting organizations of this part. China Food and Drug Administration
Hangzhou Medical Device Quality Supervision and Inspection Center.
The main drafters of this part. Yan Qinglai, Mao Xinxin, Jia Xiaohang, He Tao,
Qi Weiming.
Medical endoscopes – Rigid endoscope –
Part 3. Marking and instruction manual
1 Scope
This part of YY 0068 specifies the requirements for the marking and instruction
manual of rigid endoscope.
2 Normative references
The provisions in following documents become the provisions of this part
through reference in this part of YY 0068. For the dated references, the
subsequent amendments (excluding corrections) or revisions do not apply to
this part; however, parties who reach an agreement based on this part are
encouraged to study if the latest versions of these documents are applicable.
For undated references, the latest edition of the referenced document applies.
GB 9706.19 Medical electrical equipment - Part 2. Particular requirements
for the safety of endoscopic equipment (GB 9706.19-2000, idt IEC
60601-2-18.1996)
YY 0068.1 Medical Endoscopes - Rigid Endoscope - Part 1. Optical
properties and test methods
YY 0068.2 Medical endoscopes - Rigid endoscope - Part 2. Mechanical
properties and test methods
ISO 10526.1999 CIE S 005 CIE Standard illuminants for colorimetry
3 Requirements
3.1 Marking
3.1.1 Minimum marking
Each endoscope shall have at least the following markings.
a) An identification number and/or other markings sufficient to identify the
endoscope and manufacturer;
Appendix A
(Informative)
Examples of instruction manual
Instruction manual
A.1 Please read the entire contents of this manual thoroughly before using the
XXX endoscope manufactured by the XXX Endoscope Co., Ltd. Failure to
follow the instructions and warnings in this manual can have serious
consequences for the patient.
A.2 Endoscopes shall only be used by practicing physicians and trained
medical professionals.
A.3 This endoscope is used for XXX surgery and diagnosis and treatment in
the surgery.
A.4 The endoscope can be used in conjunction with the video and light source
equipment to observe the body cavity situation.
Correct use method. First MAKE sure that the endoscope is properly cleaned,
disinfected and/or sterilized before use (refer to the cleaning, sterilizing and
disinfection parts for detailed procedures); CHECK the endoscope for damage
and normal function; FOLLOW all the manufacturer's instructions to operate all
video and light source equipment; CONNECT the fiber optic cable to the fiber
optic cable interface on the endoscope; FOLLOW the appropriate medical
surgical code, and REFER to the appropriate surgical literatures for the
endoscopic surgical entry points.
A.5 Endoscope identification and parameters, including the contents in Table A.
1.
Table A.1 -- Identification and parameters
No. Items
Manufacturer's name. xx endoscope Co., Ltd.
Manufacturer’s address. No. XXX XXX road, XXX economic development zone, XXX city, XXX
province
2 Name. xx endoscope Identification number. XXXXXX
3 Insertion part maximum width. 4 mm (round) Working length. 200 mm
4 The minimum width of the instrument channel. /
A.7 Applicable instructions. The endoscope can be used in conjunction with
various camera systems, cold light sources, high-frequency generators, and
high-frequency equipment which comply with GB 9706.19.
WARNING. The minimum required light intensity for the light source to achieve
the best endoscopic field of view illumination is always adjusted, either for
direct or video use. The higher the light intensity of the light source, the greater
the amount of heat generated at the end of the endoscope.
A.8 The inspection instructions on the endoscope to be reasonably
guaranteed by following the operating procedures.
Check the products. Visual inspection of the product to ensure that it is no rust,
no indentation, and no scratches.
Check the image quality. through the optical tube, it shall be able to clearly
view the text about 30 mm away from the end of the objective lens.
A.9 Cleaning instructions on the reusable endoscope
A.9.1 After use, it shall be thoroughly cleaned by flowing water to remove the
residual substances such as blood and mucus, AND wipe it dry.
A.9.2 After drying the endoscope, it is placed in the multi-enzyme washing
solution for soaking.
A.9.3 Thoroughly CLEAN the endoscopic components, the tube inside shall be
thoroughly washed with a high-pressure water-gun, the removable parts must
be disassembled and cleaned, AND cleaned with an ultrasonic cleaner for 5
min ~ 10 min.
A.9.4 USE a soft brush to thoroughly brush the tube inside, AND pay attention
to avoid scratching the lens surface.
A.9.5 Instructions for special cleaning tools or equipment
It can only use the ultrasonic cleaner that the manufacturer of the cleaning
machine manufactures to clean the endoscope for cleaning the endoscope.
For the cleaning process, REFER to the manual of the ultrasonic cleaner.
A.10 Instruction of endoscope’s bearable special disinfection and sterilization
environment
A.10.1 Disinfection instructions
Endoscopes can be chemically sterilized using advanced disinfection solutions
containing 2% glutaraldehyde specifically for endoscope. It shall avoid using
Share