1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY 0174-2019 English PDF
YY 0174-2019 English PDF
Regular price
$230.00 USD
Regular price
Sale price
$230.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY 0174-2019 (Self-service in 1-minute)
Historical versions (Master-website): YY 0174-2019
Preview True-PDF (Reload/Scroll-down if blank)
YY 0174-2019: [YY/T 0174-2019] Scalpel blade
YY 0174-2019
Scalpel blade
ICS 11.040.30
C31
People's Republic of China Pharmaceutical Industry Standards
Replace YY 0174-2005
Surgical blade
2019-05-31 released
2020-06-01 Implementation
Issued by the National Medical Products Administration
Preface
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
This standard replaces YY 0174-2005 "Surgery Blade".
Compared with YY 0174-2005, the main changes of this standard are as follows.
---Revised the scope of application (see Chapter 1, Chapter 1 of the.2005 edition);
---Revised manufacturing materials (see 3.3, 3.2 of the.2005 edition);
---Added the blade identification requirements (see 3.4);
---Revised the hardness requirements (see 4.4, 4.4.1 of the.2005 edition);
---Added anti-rust performance requirements (see 4.6);
---Added ethylene oxide residue requirements (see 4.8);
---Added requirements for packaging labels and instructions (see 4.9);
---Revised the surface roughness test method (see 5.2.1, 5.2.1 of the.2005 edition);
---Revised the cutting edge sharpness test method (see 5.3, 5.3 of the.2005 edition);
---Revised the type inspection (see Chapter 6, Chapter 6 of the.2005 edition);
---Revised the labeling and instructions requirements (see Chapter 7, Chapter 7 of the.2005 edition);
---Revised the packaging, transportation and storage requirements (see Chapter 8, Chapter 8 of the.2005 edition).
Please note that some of the contents of this document may involve patents, and the issuing agency of this document does not bear the responsibility for identifying these patents.
This standard was proposed by the State Drug Administration.
This standard is under the jurisdiction of the National Technical Committee for Standardization of Surgical Instruments (SAC/TC94).
Drafting organizations of this standard. Shanghai Pudong Jinhuan Medical Products Co., Ltd., Shanghai Medical Device Testing Institute, Huaiyin Medical Devices Co., Ltd.
Limited company.
The main drafters of this standard. Zhang Yanqing, Wang Zewei, Gao Bai, Wang Fengcai, Huang Shuze, Lu Guangheng, Lu Lidong.
The previous versions of the standard replaced by this standard are as follows.
---WS2/Z45~WS2/Z46-1982;
---GB n210-1983;
---GB 2544-1988;
---YY/T 0174-1994, YY 0293-1997;
---YY 0174-2005.
Surgical blade
1 Scope
This standard specifies the classification and identification, requirements, test methods, type inspection, labeling and instructions, packaging, transportation, and storage of surgical blades.
And expiry date.
This standard applies to surgical blades (hereinafter referred to as blades) installed on the scalpel handle for cutting soft tissues.
2 Normative references
The following documents are indispensable for the application of this document. For dated reference documents, only the dated version applies to this article
Pieces. For undated reference documents, the latest version (including all amendments) is applicable to this document.
GB/T 191 Packaging, storage and transportation pictorial signs
GB/T 1299 Tool and Die Steel
GB/T 4340.1 Vickers hardness test of metallic materials Part 1.Test method
GB 8662 Matching size of scalpel blade and scalpel handle
GB/T 9969 General Rules for the Use of Industrial Products
GB/T 14233.1 Medical infusion, blood transfusion, and injection equipment inspection methods Part 1.Chemical analysis methods
YY 0167-2005 Non-absorbable surgical suture
YY/T 0294.1 Metallic materials for surgical instruments Part 1.Stainless steel
YY/T 0466.1 Medical devices are used for medical device labeling, marking and providing information symbols. Part 1.General requirements
YY/T 1052 Surgical instrument mark
Pharmacopoeia of the People's Republic of China (2015 Edition·Fourth Edition)
3 Classification and identification
3.1 Delivery status
The blade has two delivery states. aseptic and non-sterile (hereinafter referred to as sterile blade and non-sterile blade).
3.2 Specifications
Please refer to Appendix A for the specifications and dimensions of the blade, and the assembly specifications of the knife handle.
3.3 Materials
The manufacturing material of the blade should be made of non-alloy tool steel that meets the requirements of GB/T 1299 or martensite that meets the requirements of YY/T 0294.1
Stainless steel, or other applicable metal materials that meet the requirements of Chapter 4.
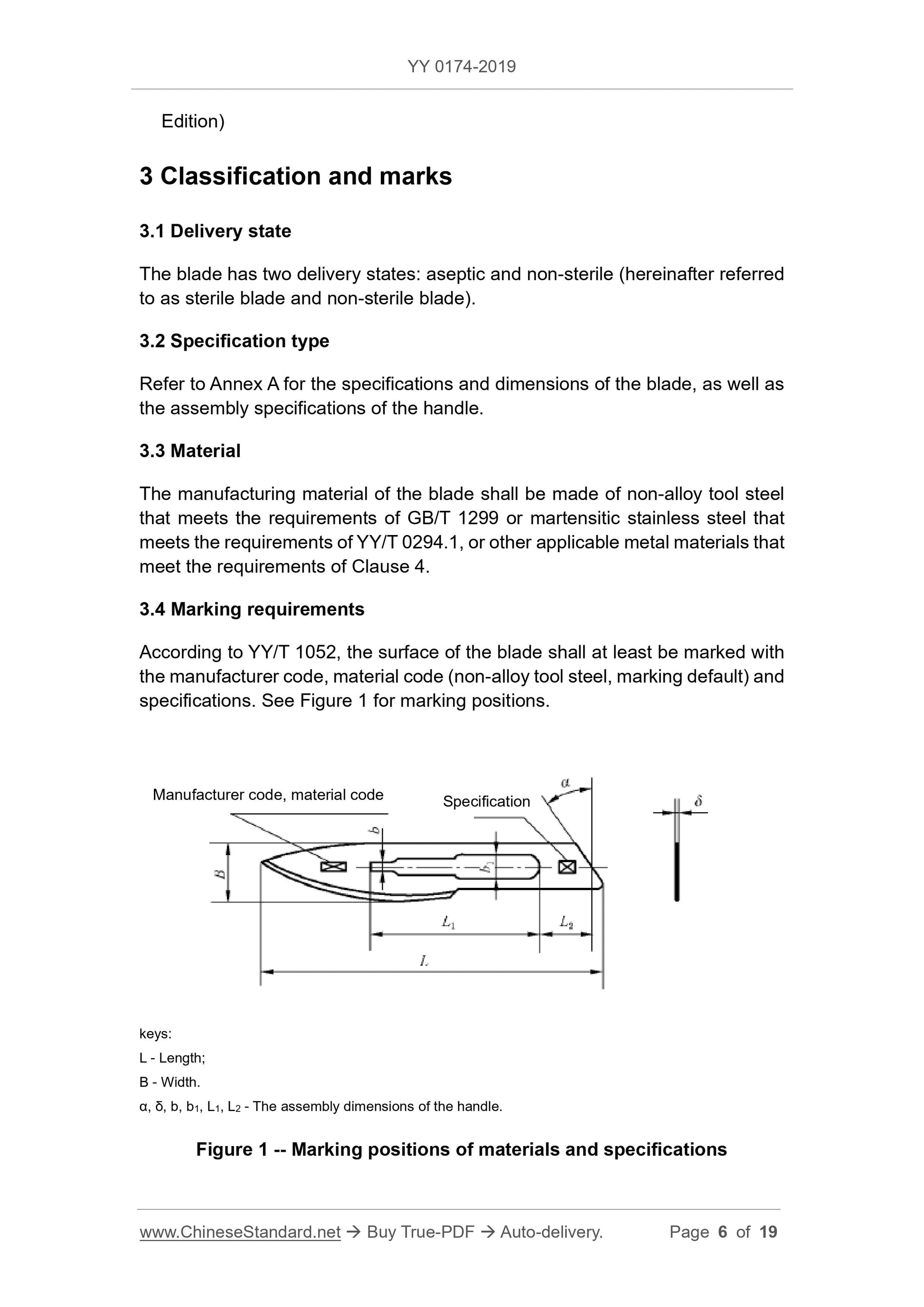
3.4 Marking requirements
According to the provisions of YY/T 1052, the surface of the blade should at least be marked with the manufacturer code, material code (non-alloy tool steel, marking default) and specification.
Grid, the location of the mark is shown in Figure 1.
4 requirements
4.1 Dimensions
The blade assembly dimensions α, δ, b, b1, L1 and L2, see Figure 1, should meet the requirements of GB 8662.
4.2 Appearance
4.2.1 The value of the surface roughness Ra of the insert should not be greater than 0.4μm, and the value of the surface roughness Ra of the cutting edge surface should not be greater than
0.8μm.
4.2.2 The cutting edge of the blade should be free of nicks, white edges, curls, cracks, etc.
4.2.3 The width of both sides of the cutting edge of the blade should be similar, and the surface should be free of focal spots.
4.2.4 The blade should be flat, and there should be no cracks, sharp edges, burrs, or obvious pitting.
4.3 Cutting edge sharpness
The cutting edge of the blade should be sharp, and its cutting force should not be greater than 0.80N.
4.4 Hardness
The blade should be heat treated, and its hardness is not less than 650HV10.
4.5 Flexibility
The blade should have good elasticity, and there should be no fracture and no obvious deformation after the test.
4.6 Anti-rust performance
The blade should have good anti-rust performance and there should be no rust spots on the surface.
4.7 Sterility
If it is a sterile blade, it should be sterilized through a confirmed sterilization process, and the blade should be sterile.
4.8 Residual ethylene oxide
If the blade is sterilized with ethylene oxide gas, the residual amount of ethylene oxide should not exceed 10μg/g.
4.9 Packaging labels and instructions
The packaging label should be clear and comply with the provisions of Chapter 7.The instructions should specify detailed sterilization or disinfection methods.
5 Test method
5.1 Dimensions
Measured with a universal measuring tool, it should meet the requirements of 4.1.
5.2 Appearance
5.2.1 Comparing with the surface roughness comparison sample block should meet the requirements of 4.2.1.
5.2.2 Visual observation should meet the requirements of 4.2.2, 4.2.3, and 4.2.4.
5.3 Cutting edge sharpness
The test method is shown in Appendix B, which should meet the requirements of 4.3.
5.4 Hardness
Carry out the test according to the method specified in GB/T 4340.1, measure three points on the blade, and take the arithmetic average value, which should meet the requirements of 4.4.
5.5 Flexibility
The test method is shown in Appendix C, which should meet the requirements of 4.5.
5.6 Anti-rust performance
5.6.1 Open the package, the surface of the blade should meet the requirements of 4.6.
5.6.2 Non-sterile blades are tested according to the sterilization or disinfection method specified in the instructions, and the surface of the blade should meet the requirements of 4.6.
5.7 Sterility
It should be carried out in accordance with the general rule 1101 of the "Pharmacopoeia of the People's Republic of China" (2015 Edition·Part Four) "Sterile Inspection Method", which should meet the requirements of 4.7.
5.8 Residual ethylene oxide
It should be carried out according to the method specified in GB/T 14233.1 and should meet the requirements in 4.8.
5.9 Packaging labels and instructions
By visual observation, the relevant content of the smallest package label and manual should meet the requirements of 4.9.
6 Type inspection
The inspection items, inspection quantity and judgment rules of the blade are shown in Table 1.
7 Labels and instructions
7.1 Label
7.1.1 The minimum package of non-sterile blades should have at least the following contents or symbols.
a) Product name, specifications, quantity and manufacturing materials;
b) The number of the filing voucher;
c) Batch code or batch number, production date, expiration date or expiration date;
d) The name, residence, and contact information of the filing person;
e) Production company name, domicile, production address, contact information and production record certificate number;
f) The words or symbols of "Other contents are detailed in the manual".
7.1.2 The minimum package of sterile blades shall have at least the following contents or symbols.
a) Product name and specifications;
b) Sterilization method, aseptic, single-use mark;
c) Product registration certificate number;
d) The words or symbols of "use if the package is damaged";
e) Batch code or batch number, production date, expiry date or expiration date;
f) The name and domicile of the registrant or manufacturer;
g) The words or symbols of "Other contents are detailed in the manual".
7.1.3 The minimum sales unit package should have at least the following contents or symbols.
a) Product name;
b) Specification and quantity;
c) Sterilization method (for sterile blades), single-use mark;
d) Batch code or batch number, production date, expiration date or expiration date;
e) Product registration certificate number or record certificate number, production license number or production record certificate number;
f) The name of the registrant or filing person, the name of the manufacturer, the address, and the contact information;
7.1.4 Each minimum sales unit shall have an inspection certificate, and the certificate shall have at least the following contents.
a) The name of the registrant or recorder or manufacturer;
b) Product technical requirement number;
c) Batch code or batch number;
d) Inspection date and inspector code.
7.1.5 The packaging, storage and transportation marks on the packaging should comply with the relevant regulations of GB/T 191 and YY/T 0466.1.
7.2 Instructions
There should be an instruction manual in each minimum sales unit, and the preparation of the instruction manual should comply with the requirements of GB/T 9969, and should at least contain the following content.
a) Product name and specifications;
b) The name, address, contact information and after-sales service information of the registrant or recorder or manufacturer.
The name, address, production license number or production record certificate number of the consigning manufacturer;
c) Product registration certificate number or record certificate number, production license number or production record certificate number;
d) Product technical requirement number;
e) Product performance, manufacturing materials, and scope of application;
f) Installation and use instructions or diagrams;
g) Storage conditions and methods;
h) One-time use, detailed sterilization or disinfection method, description of production date, expiration date or expiration date;
i) Explanation of graphics, symbols, abbreviations, etc. used in the label;
j) The date of preparation or revision of the manual;
k) Contraindications, precautions, warnings and reminders.
1) Requirements to ensure the correct and safe use of blades, and post-processing requirements for safe use;
2) Matters needing attention when the blade is used in conjunction with other instruments;
3) The sterile blade should indicate the treatment method when the smallest single package is damaged.
8 Packaging, transportation, storage and expiry date
8.1 The initial packaging consists of a single sterile blade or multiple non-sterile blades, and the initial packaging should be the smallest package.
Note. The rust-proof paper packaging of a single non-sterile blade is not regarded as the minimum packaging.
8.2 The middle package is composed of one or more small packages, and the middle package should be the smallest sales unit.
8.3 After the initial packaging of the sterile blade is opened, there should be traces of the opening.
8.4 The smallest sales unit should be accompanied by instructions and certificates.
8.5 Under the transportation conditions specified by the manufacturer, the packaging is undamaged, and the writing on the packaging label should be clear within the validity period.
8.6 The packaged blades should be stored in a room temperature, clean, well-ventilated, and non-corrosive gas environment, and the expiration date should not be less than 2 years.
B.3 Test materials
The test material adopts 3-0 uncoated 3-0 suture with twisted strands of class I silk in accordance with YY 0167-2005.
B.4 Test method
B.4.1 The surface of the sample (blade) should be cleaned of grease and care should be taken to maintain the quality of the original product.
B.4.2 Test procedure.
---Fix the sample (blade) on the fixture;
---Fix one end of the suture thread on a winding clamp, and add 100g weight to the other end of the suture thread to make the suture thread horizontal and secure
Set it on the other winding clamp, remove the weight, and prepare for the test;
---The sample moves vertically downward at a speed not greater than 10mm/s, and cuts the middle part of the suture line;
---When the suture is cut, record the test value.
B.4.3 For each blade, measure the front, middle, and back three points of the blade according to the test procedure B.4.2, and take the arithmetic average of the three-point test values as the test
Get Quotation: Click YY 0174-2019 (Self-service in 1-minute)
Historical versions (Master-website): YY 0174-2019
Preview True-PDF (Reload/Scroll-down if blank)
YY 0174-2019: [YY/T 0174-2019] Scalpel blade
YY 0174-2019
Scalpel blade
ICS 11.040.30
C31
People's Republic of China Pharmaceutical Industry Standards
Replace YY 0174-2005
Surgical blade
2019-05-31 released
2020-06-01 Implementation
Issued by the National Medical Products Administration
Preface
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
This standard replaces YY 0174-2005 "Surgery Blade".
Compared with YY 0174-2005, the main changes of this standard are as follows.
---Revised the scope of application (see Chapter 1, Chapter 1 of the.2005 edition);
---Revised manufacturing materials (see 3.3, 3.2 of the.2005 edition);
---Added the blade identification requirements (see 3.4);
---Revised the hardness requirements (see 4.4, 4.4.1 of the.2005 edition);
---Added anti-rust performance requirements (see 4.6);
---Added ethylene oxide residue requirements (see 4.8);
---Added requirements for packaging labels and instructions (see 4.9);
---Revised the surface roughness test method (see 5.2.1, 5.2.1 of the.2005 edition);
---Revised the cutting edge sharpness test method (see 5.3, 5.3 of the.2005 edition);
---Revised the type inspection (see Chapter 6, Chapter 6 of the.2005 edition);
---Revised the labeling and instructions requirements (see Chapter 7, Chapter 7 of the.2005 edition);
---Revised the packaging, transportation and storage requirements (see Chapter 8, Chapter 8 of the.2005 edition).
Please note that some of the contents of this document may involve patents, and the issuing agency of this document does not bear the responsibility for identifying these patents.
This standard was proposed by the State Drug Administration.
This standard is under the jurisdiction of the National Technical Committee for Standardization of Surgical Instruments (SAC/TC94).
Drafting organizations of this standard. Shanghai Pudong Jinhuan Medical Products Co., Ltd., Shanghai Medical Device Testing Institute, Huaiyin Medical Devices Co., Ltd.
Limited company.
The main drafters of this standard. Zhang Yanqing, Wang Zewei, Gao Bai, Wang Fengcai, Huang Shuze, Lu Guangheng, Lu Lidong.
The previous versions of the standard replaced by this standard are as follows.
---WS2/Z45~WS2/Z46-1982;
---GB n210-1983;
---GB 2544-1988;
---YY/T 0174-1994, YY 0293-1997;
---YY 0174-2005.
Surgical blade
1 Scope
This standard specifies the classification and identification, requirements, test methods, type inspection, labeling and instructions, packaging, transportation, and storage of surgical blades.
And expiry date.
This standard applies to surgical blades (hereinafter referred to as blades) installed on the scalpel handle for cutting soft tissues.
2 Normative references
The following documents are indispensable for the application of this document. For dated reference documents, only the dated version applies to this article
Pieces. For undated reference documents, the latest version (including all amendments) is applicable to this document.
GB/T 191 Packaging, storage and transportation pictorial signs
GB/T 1299 Tool and Die Steel
GB/T 4340.1 Vickers hardness test of metallic materials Part 1.Test method
GB 8662 Matching size of scalpel blade and scalpel handle
GB/T 9969 General Rules for the Use of Industrial Products
GB/T 14233.1 Medical infusion, blood transfusion, and injection equipment inspection methods Part 1.Chemical analysis methods
YY 0167-2005 Non-absorbable surgical suture
YY/T 0294.1 Metallic materials for surgical instruments Part 1.Stainless steel
YY/T 0466.1 Medical devices are used for medical device labeling, marking and providing information symbols. Part 1.General requirements
YY/T 1052 Surgical instrument mark
Pharmacopoeia of the People's Republic of China (2015 Edition·Fourth Edition)
3 Classification and identification
3.1 Delivery status
The blade has two delivery states. aseptic and non-sterile (hereinafter referred to as sterile blade and non-sterile blade).
3.2 Specifications
Please refer to Appendix A for the specifications and dimensions of the blade, and the assembly specifications of the knife handle.
3.3 Materials
The manufacturing material of the blade should be made of non-alloy tool steel that meets the requirements of GB/T 1299 or martensite that meets the requirements of YY/T 0294.1
Stainless steel, or other applicable metal materials that meet the requirements of Chapter 4.
3.4 Marking requirements
According to the provisions of YY/T 1052, the surface of the blade should at least be marked with the manufacturer code, material code (non-alloy tool steel, marking default) and specification.
Grid, the location of the mark is shown in Figure 1.
4 requirements
4.1 Dimensions
The blade assembly dimensions α, δ, b, b1, L1 and L2, see Figure 1, should meet the requirements of GB 8662.
4.2 Appearance
4.2.1 The value of the surface roughness Ra of the insert should not be greater than 0.4μm, and the value of the surface roughness Ra of the cutting edge surface should not be greater than
0.8μm.
4.2.2 The cutting edge of the blade should be free of nicks, white edges, curls, cracks, etc.
4.2.3 The width of both sides of the cutting edge of the blade should be similar, and the surface should be free of focal spots.
4.2.4 The blade should be flat, and there should be no cracks, sharp edges, burrs, or obvious pitting.
4.3 Cutting edge sharpness
The cutting edge of the blade should be sharp, and its cutting force should not be greater than 0.80N.
4.4 Hardness
The blade should be heat treated, and its hardness is not less than 650HV10.
4.5 Flexibility
The blade should have good elasticity, and there should be no fracture and no obvious deformation after the test.
4.6 Anti-rust performance
The blade should have good anti-rust performance and there should be no rust spots on the surface.
4.7 Sterility
If it is a sterile blade, it should be sterilized through a confirmed sterilization process, and the blade should be sterile.
4.8 Residual ethylene oxide
If the blade is sterilized with ethylene oxide gas, the residual amount of ethylene oxide should not exceed 10μg/g.
4.9 Packaging labels and instructions
The packaging label should be clear and comply with the provisions of Chapter 7.The instructions should specify detailed sterilization or disinfection methods.
5 Test method
5.1 Dimensions
Measured with a universal measuring tool, it should meet the requirements of 4.1.
5.2 Appearance
5.2.1 Comparing with the surface roughness comparison sample block should meet the requirements of 4.2.1.
5.2.2 Visual observation should meet the requirements of 4.2.2, 4.2.3, and 4.2.4.
5.3 Cutting edge sharpness
The test method is shown in Appendix B, which should meet the requirements of 4.3.
5.4 Hardness
Carry out the test according to the method specified in GB/T 4340.1, measure three points on the blade, and take the arithmetic average value, which should meet the requirements of 4.4.
5.5 Flexibility
The test method is shown in Appendix C, which should meet the requirements of 4.5.
5.6 Anti-rust performance
5.6.1 Open the package, the surface of the blade should meet the requirements of 4.6.
5.6.2 Non-sterile blades are tested according to the sterilization or disinfection method specified in the instructions, and the surface of the blade should meet the requirements of 4.6.
5.7 Sterility
It should be carried out in accordance with the general rule 1101 of the "Pharmacopoeia of the People's Republic of China" (2015 Edition·Part Four) "Sterile Inspection Method", which should meet the requirements of 4.7.
5.8 Residual ethylene oxide
It should be carried out according to the method specified in GB/T 14233.1 and should meet the requirements in 4.8.
5.9 Packaging labels and instructions
By visual observation, the relevant content of the smallest package label and manual should meet the requirements of 4.9.
6 Type inspection
The inspection items, inspection quantity and judgment rules of the blade are shown in Table 1.
7 Labels and instructions
7.1 Label
7.1.1 The minimum package of non-sterile blades should have at least the following contents or symbols.
a) Product name, specifications, quantity and manufacturing materials;
b) The number of the filing voucher;
c) Batch code or batch number, production date, expiration date or expiration date;
d) The name, residence, and contact information of the filing person;
e) Production company name, domicile, production address, contact information and production record certificate number;
f) The words or symbols of "Other contents are detailed in the manual".
7.1.2 The minimum package of sterile blades shall have at least the following contents or symbols.
a) Product name and specifications;
b) Sterilization method, aseptic, single-use mark;
c) Product registration certificate number;
d) The words or symbols of "use if the package is damaged";
e) Batch code or batch number, production date, expiry date or expiration date;
f) The name and domicile of the registrant or manufacturer;
g) The words or symbols of "Other contents are detailed in the manual".
7.1.3 The minimum sales unit package should have at least the following contents or symbols.
a) Product name;
b) Specification and quantity;
c) Sterilization method (for sterile blades), single-use mark;
d) Batch code or batch number, production date, expiration date or expiration date;
e) Product registration certificate number or record certificate number, production license number or production record certificate number;
f) The name of the registrant or filing person, the name of the manufacturer, the address, and the contact information;
7.1.4 Each minimum sales unit shall have an inspection certificate, and the certificate shall have at least the following contents.
a) The name of the registrant or recorder or manufacturer;
b) Product technical requirement number;
c) Batch code or batch number;
d) Inspection date and inspector code.
7.1.5 The packaging, storage and transportation marks on the packaging should comply with the relevant regulations of GB/T 191 and YY/T 0466.1.
7.2 Instructions
There should be an instruction manual in each minimum sales unit, and the preparation of the instruction manual should comply with the requirements of GB/T 9969, and should at least contain the following content.
a) Product name and specifications;
b) The name, address, contact information and after-sales service information of the registrant or recorder or manufacturer.
The name, address, production license number or production record certificate number of the consigning manufacturer;
c) Product registration certificate number or record certificate number, production license number or production record certificate number;
d) Product technical requirement number;
e) Product performance, manufacturing materials, and scope of application;
f) Installation and use instructions or diagrams;
g) Storage conditions and methods;
h) One-time use, detailed sterilization or disinfection method, description of production date, expiration date or expiration date;
i) Explanation of graphics, symbols, abbreviations, etc. used in the label;
j) The date of preparation or revision of the manual;
k) Contraindications, precautions, warnings and reminders.
1) Requirements to ensure the correct and safe use of blades, and post-processing requirements for safe use;
2) Matters needing attention when the blade is used in conjunction with other instruments;
3) The sterile blade should indicate the treatment method when the smallest single package is damaged.
8 Packaging, transportation, storage and expiry date
8.1 The initial packaging consists of a single sterile blade or multiple non-sterile blades, and the initial packaging should be the smallest package.
Note. The rust-proof paper packaging of a single non-sterile blade is not regarded as the minimum packaging.
8.2 The middle package is composed of one or more small packages, and the middle package should be the smallest sales unit.
8.3 After the initial packaging of the sterile blade is opened, there should be traces of the opening.
8.4 The smallest sales unit should be accompanied by instructions and certificates.
8.5 Under the transportation conditions specified by the manufacturer, the packaging is undamaged, and the writing on the packaging label should be clear within the validity period.
8.6 The packaged blades should be stored in a room temperature, clean, well-ventilated, and non-corrosive gas environment, and the expiration date should not be less than 2 years.
B.3 Test materials
The test material adopts 3-0 uncoated 3-0 suture with twisted strands of class I silk in accordance with YY 0167-2005.
B.4 Test method
B.4.1 The surface of the sample (blade) should be cleaned of grease and care should be taken to maintain the quality of the original product.
B.4.2 Test procedure.
---Fix the sample (blade) on the fixture;
---Fix one end of the suture thread on a winding clamp, and add 100g weight to the other end of the suture thread to make the suture thread horizontal and secure
Set it on the other winding clamp, remove the weight, and prepare for the test;
---The sample moves vertically downward at a speed not greater than 10mm/s, and cuts the middle part of the suture line;
---When the suture is cut, record the test value.
B.4.3 For each blade, measure the front, middle, and back three points of the blade according to the test procedure B.4.2, and take the arithmetic average of the three-point test values as the test
Share