1
/
of
7
www.ChineseStandard.us -- Field Test Asia Pte. Ltd.
YY 0285.3-2017 English PDF
YY 0285.3-2017 English PDF
Regular price
$145.00
Regular price
Sale price
$145.00
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
YY 0285.3-2017: [Including 2019XG1] Intravascular catheters - Sterile and single-use catheters - Part 3: Central venous catheters
Delivery: 9 seconds. Download (& Email) true-PDF + Invoice.
Get Quotation: Click YY 0285.3-2017 (Self-service in 1-minute)
Historical versions (Master-website): YY 0285.3-2017
Preview True-PDF (Reload/Scroll-down if blank)
YY 0285.3-2017
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.20
C 31
Replacing YY 0285.3-1999
Intravascular catheters - Sterile and single-use
catheters - Part 3. Central venous catheter
(ISO 10555-3.2013, Intravascular catheters - Sterile and single-use catheters
- Part 3. Central venous catheters, MOD)
ISSUED ON. JULY 17, 2017
IMPLEMENTED ON. SEPTEMBER 1, 2019
Issued by. China Food and Drug Administration
Table of Contents
Foreword ... 3
1 Scope ... 5
2 Normative references ... 5
3 Terms and definitions ... 5
4 Requirements ... 5
Foreword
YY 0285 "Intravascular catheters - Sterile and single-use catheters" consists of
four parts.
- Part 1. General requirements;
- Part 3. Central venous catheters;
- Part 4. Balloon dilatation catheters;
- Part 5. Over-needle peripheral catheters.
This Part is Part 3 of YY 0285.
This Part was drafted in accordance with the rules given in GB/T 1.1-2009.
This Part replaces YY 0285.3-1999 "Sterile single-use intravascular catheters -
Part 3. Central venous catheters". Compared with YY 0285.3-1999, the main
technical changes are as follows.
- updated the normative references;
- deleted Annex A.
This Part uses redrafting method to modify and adopt ISO 10555-3.2013
"Intravascular catheters - Sterile and single-use catheters - Part 3. Central
venous catheters".
The technical differences and reasons between this Part and ISO 10555-3.2013
are as follows.
- about normative references, this Standard made an adjustment on
technical differences so as to adapt to the technical conditions in China;
see Clause 2 "Normative references" for the adjustment, as follows.
● replaced ISO 10555-1.2013 with YY 0285.1-2017 that modified and
adopted the International standard.
Attention is drawn to the possibility that some of the elements of this document
may be the subject of patent rights. The issuing authority shall not be held
responsible for identifying any or all such patent rights.
This Part was proposed by China Food and Drug Administration.
This Part shall be under the jurisdiction of National Technical Committee on
Medical Infusion Apparatus of China (SAC/TC 106).
Intravascular catheters - Sterile and single-use
catheters - Part 3. Central venous catheter
1 Scope
This Part specifies the requirements for sterile and single-use central venous
catheter.
2 Normative references
The following referenced documents are indispensable for the application of
this document. For dated references, only the edition cited applies. For undated
references, the latest edition of the referenced document (including any
amendments) applies.
YY 0285.1-2017, Intravascular catheters - Sterile and single-use catheters -
Part 1. General requirements (ISO 10555-1.2013, MOD)
3 Terms and definitions
For the purposes of this document, the terms and definitions defined in YY
0285.1-2017 and the followings apply.
3.1 central venous catheter
a central venous system is inserted for intravascular catheters for the delivery
of fluids or blood samples and/or for pressure or other measurements, and is
divided into single chambers or multiple chambers
NOTE The catheter may have a fixation system as an integral part of the instrument.
4 Requirements
4.1 General
The catheter shall comply with the requirements of YY 0285.1-2017 except for
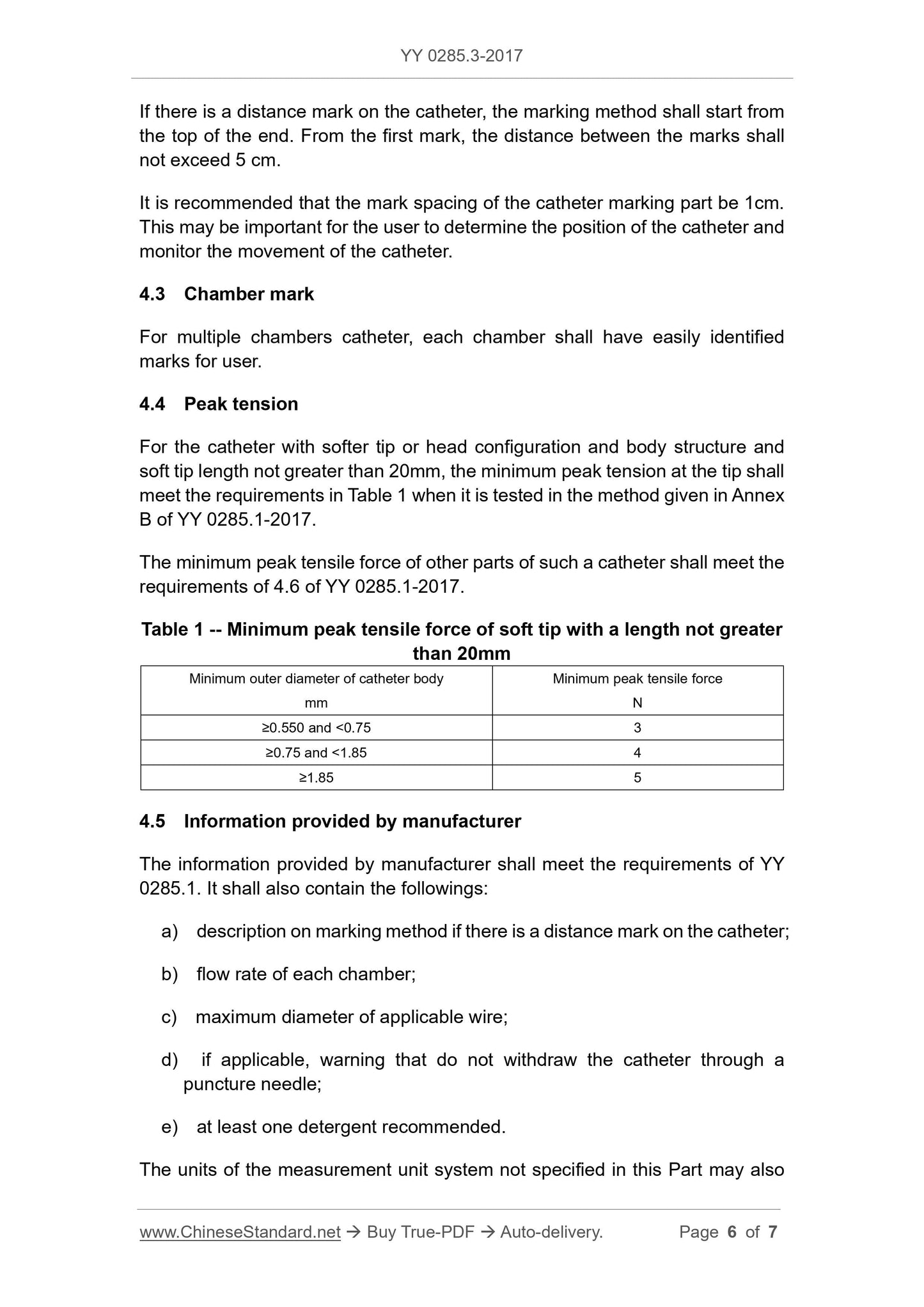
peak tension (see YY 0285.1-2017, 4.6). The peak tension shall meet the
requirements of 4.4.
4.2 Distance mark
YY 0285.3-2017
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.20
C 31
Replacing YY 0285.3-1999
Intravascular catheters - Sterile and single-use
catheters - Part 3. Central venous catheter
(ISO 10555-3.2013, Intravascular catheters - Sterile and single-use catheters
- Part 3. Central venous catheters, MOD)
ISSUED ON. JULY 17, 2017
IMPLEMENTED ON. SEPTEMBER 1, 2019
Issued by. China Food and Drug Administration
Table of Contents
Foreword ... 3
1 Scope ... 5
2 Normative references ... 5
3 Terms and definitions ... 5
4 Requirements ... 5
Foreword
YY 0285 "Intravascular catheters - Sterile and single-use catheters" consists of
four parts.
- Part 1. General requirements;
- Part 3. Central venous catheters;
- Part 4. Balloon dilatation catheters;
- Part 5. Over-needle peripheral catheters.
This Part is Part 3 of YY 0285.
This Part was drafted in accordance with the rules given in GB/T 1.1-2009.
This Part replaces YY 0285.3-1999 "Sterile single-use intravascular catheters -
Part 3. Central venous catheters". Compared with YY 0285.3-1999, the main
technical changes are as follows.
- updated the normative references;
- deleted Annex A.
This Part uses redrafting method to modify and adopt ISO 10555-3.2013
"Intravascular catheters - Sterile and single-use catheters - Part 3. Central
venous catheters".
The technical differences and reasons between this Part and ISO 10555-3.2013
are as follows.
- about normative references, this Standard made an adjustment on
technical differences so as to adapt to the technical conditions in China;
see Clause 2 "Normative references" for the adjustment, as follows.
● replaced ISO 10555-1.2013 with YY 0285.1-2017 that modified and
adopted the International standard.
Attention is drawn to the possibility that some of the elements of this document
may be the subject of patent rights. The issuing authority shall not be held
responsible for identifying any or all such patent rights.
This Part was proposed by China Food and Drug Administration.
This Part shall be under the jurisdiction of National Technical Committee on
Medical Infusion Apparatus of China (SAC/TC 106).
Intravascular catheters - Sterile and single-use
catheters - Part 3. Central venous catheter
1 Scope
This Part specifies the requirements for sterile and single-use central venous
catheter.
2 Normative references
The following referenced documents are indispensable for the application of
this document. For dated references, only the edition cited applies. For undated
references, the latest edition of the referenced document (including any
amendments) applies.
YY 0285.1-2017, Intravascular catheters - Sterile and single-use catheters -
Part 1. General requirements (ISO 10555-1.2013, MOD)
3 Terms and definitions
For the purposes of this document, the terms and definitions defined in YY
0285.1-2017 and the followings apply.
3.1 central venous catheter
a central venous system is inserted for intravascular catheters for the delivery
of fluids or blood samples and/or for pressure or other measurements, and is
divided into single chambers or multiple chambers
NOTE The catheter may have a fixation system as an integral part of the instrument.
4 Requirements
4.1 General
The catheter shall comply with the requirements of YY 0285.1-2017 except for
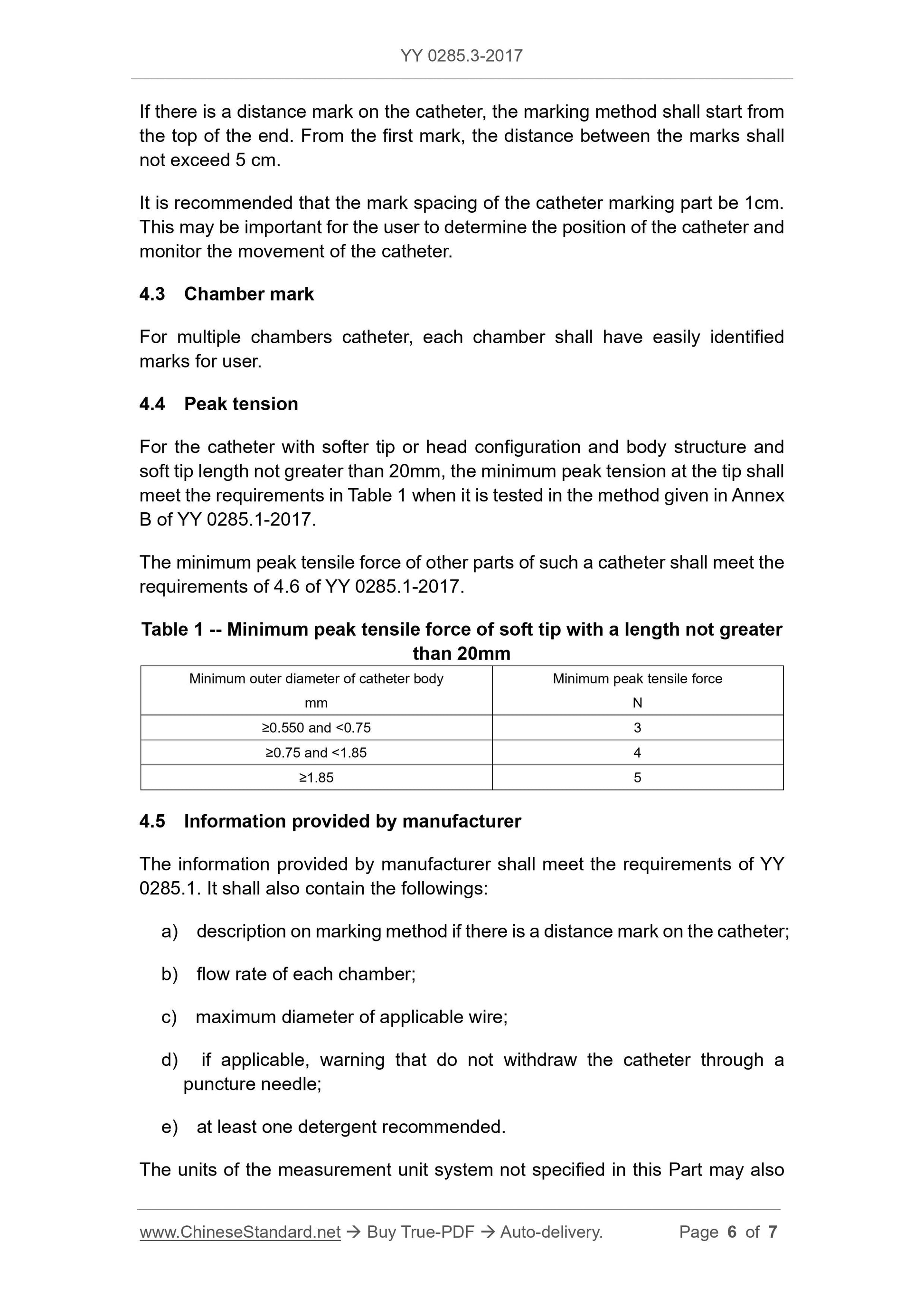
peak tension (see YY 0285.1-2017, 4.6). The peak tension shall meet the
requirements of 4.4.
4.2 Distance mark
Delivery: 9 seconds. Download (& Email) true-PDF + Invoice.
Get Quotation: Click YY 0285.3-2017 (Self-service in 1-minute)
Historical versions (Master-website): YY 0285.3-2017
Preview True-PDF (Reload/Scroll-down if blank)
YY 0285.3-2017
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.20
C 31
Replacing YY 0285.3-1999
Intravascular catheters - Sterile and single-use
catheters - Part 3. Central venous catheter
(ISO 10555-3.2013, Intravascular catheters - Sterile and single-use catheters
- Part 3. Central venous catheters, MOD)
ISSUED ON. JULY 17, 2017
IMPLEMENTED ON. SEPTEMBER 1, 2019
Issued by. China Food and Drug Administration
Table of Contents
Foreword ... 3
1 Scope ... 5
2 Normative references ... 5
3 Terms and definitions ... 5
4 Requirements ... 5
Foreword
YY 0285 "Intravascular catheters - Sterile and single-use catheters" consists of
four parts.
- Part 1. General requirements;
- Part 3. Central venous catheters;
- Part 4. Balloon dilatation catheters;
- Part 5. Over-needle peripheral catheters.
This Part is Part 3 of YY 0285.
This Part was drafted in accordance with the rules given in GB/T 1.1-2009.
This Part replaces YY 0285.3-1999 "Sterile single-use intravascular catheters -
Part 3. Central venous catheters". Compared with YY 0285.3-1999, the main
technical changes are as follows.
- updated the normative references;
- deleted Annex A.
This Part uses redrafting method to modify and adopt ISO 10555-3.2013
"Intravascular catheters - Sterile and single-use catheters - Part 3. Central
venous catheters".
The technical differences and reasons between this Part and ISO 10555-3.2013
are as follows.
- about normative references, this Standard made an adjustment on
technical differences so as to adapt to the technical conditions in China;
see Clause 2 "Normative references" for the adjustment, as follows.
● replaced ISO 10555-1.2013 with YY 0285.1-2017 that modified and
adopted the International standard.
Attention is drawn to the possibility that some of the elements of this document
may be the subject of patent rights. The issuing authority shall not be held
responsible for identifying any or all such patent rights.
This Part was proposed by China Food and Drug Administration.
This Part shall be under the jurisdiction of National Technical Committee on
Medical Infusion Apparatus of China (SAC/TC 106).
Intravascular catheters - Sterile and single-use
catheters - Part 3. Central venous catheter
1 Scope
This Part specifies the requirements for sterile and single-use central venous
catheter.
2 Normative references
The following referenced documents are indispensable for the application of
this document. For dated references, only the edition cited applies. For undated
references, the latest edition of the referenced document (including any
amendments) applies.
YY 0285.1-2017, Intravascular catheters - Sterile and single-use catheters -
Part 1. General requirements (ISO 10555-1.2013, MOD)
3 Terms and definitions
For the purposes of this document, the terms and definitions defined in YY
0285.1-2017 and the followings apply.
3.1 central venous catheter
a central venous system is inserted for intravascular catheters for the delivery
of fluids or blood samples and/or for pressure or other measurements, and is
divided into single chambers or multiple chambers
NOTE The catheter may have a fixation system as an integral part of the instrument.
4 Requirements
4.1 General
The catheter shall comply with the requirements of YY 0285.1-2017 except for
peak tension (see YY 0285.1-2017, 4.6). The peak tension shall meet the
requirements of 4.4.
4.2 Distance mark
YY 0285.3-2017
YY
PHARMACEUTICAL INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.20
C 31
Replacing YY 0285.3-1999
Intravascular catheters - Sterile and single-use
catheters - Part 3. Central venous catheter
(ISO 10555-3.2013, Intravascular catheters - Sterile and single-use catheters
- Part 3. Central venous catheters, MOD)
ISSUED ON. JULY 17, 2017
IMPLEMENTED ON. SEPTEMBER 1, 2019
Issued by. China Food and Drug Administration
Table of Contents
Foreword ... 3
1 Scope ... 5
2 Normative references ... 5
3 Terms and definitions ... 5
4 Requirements ... 5
Foreword
YY 0285 "Intravascular catheters - Sterile and single-use catheters" consists of
four parts.
- Part 1. General requirements;
- Part 3. Central venous catheters;
- Part 4. Balloon dilatation catheters;
- Part 5. Over-needle peripheral catheters.
This Part is Part 3 of YY 0285.
This Part was drafted in accordance with the rules given in GB/T 1.1-2009.
This Part replaces YY 0285.3-1999 "Sterile single-use intravascular catheters -
Part 3. Central venous catheters". Compared with YY 0285.3-1999, the main
technical changes are as follows.
- updated the normative references;
- deleted Annex A.
This Part uses redrafting method to modify and adopt ISO 10555-3.2013
"Intravascular catheters - Sterile and single-use catheters - Part 3. Central
venous catheters".
The technical differences and reasons between this Part and ISO 10555-3.2013
are as follows.
- about normative references, this Standard made an adjustment on
technical differences so as to adapt to the technical conditions in China;
see Clause 2 "Normative references" for the adjustment, as follows.
● replaced ISO 10555-1.2013 with YY 0285.1-2017 that modified and
adopted the International standard.
Attention is drawn to the possibility that some of the elements of this document
may be the subject of patent rights. The issuing authority shall not be held
responsible for identifying any or all such patent rights.
This Part was proposed by China Food and Drug Administration.
This Part shall be under the jurisdiction of National Technical Committee on
Medical Infusion Apparatus of China (SAC/TC 106).
Intravascular catheters - Sterile and single-use
catheters - Part 3. Central venous catheter
1 Scope
This Part specifies the requirements for sterile and single-use central venous
catheter.
2 Normative references
The following referenced documents are indispensable for the application of
this document. For dated references, only the edition cited applies. For undated
references, the latest edition of the referenced document (including any
amendments) applies.
YY 0285.1-2017, Intravascular catheters - Sterile and single-use catheters -
Part 1. General requirements (ISO 10555-1.2013, MOD)
3 Terms and definitions
For the purposes of this document, the terms and definitions defined in YY
0285.1-2017 and the followings apply.
3.1 central venous catheter
a central venous system is inserted for intravascular catheters for the delivery
of fluids or blood samples and/or for pressure or other measurements, and is
divided into single chambers or multiple chambers
NOTE The catheter may have a fixation system as an integral part of the instrument.
4 Requirements
4.1 General
The catheter shall comply with the requirements of YY 0285.1-2017 except for
peak tension (see YY 0285.1-2017, 4.6). The peak tension shall meet the
requirements of 4.4.
4.2 Distance mark
Share