1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY 0286.3-2017 English PDF
YY 0286.3-2017 English PDF
Regular price
$150.00 USD
Regular price
Sale price
$150.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY 0286.3-2017 (Self-service in 1-minute)
Historical versions (Master-website): YY 0286.3-2017
Preview True-PDF (Reload/Scroll-down if blank)
YY 0286.3-2017: Special infusion sets - Part 3: Light-resistant infusion sets for single use
YY 0286.3-2017
Special infusion sets -- Part 3. Light-resistant infusion sets for single use
ICS 11.040.20
C31
People's Republic of China Pharmaceutical Industry Standard
Special infusion set
Part 3. Disposable light infusion sets
Part 3. Light-resistantinfusionsetsforsingleuse
Released on.2017-07-17
2019-01-01 implementation
State Food and Drug Administration issued
Content
Foreword I
Introduction II
1 range 1
2 Normative references 1
3 General requirements 1
4 material 1
5 Physical requirements 1
6 Chemical requirements 1
7 Biological requirements 2
8 logo 2
9 Pack 2
10 type test 2
Appendix A (Normative Appendix) Determination of Transmittance 3
Appendix B (Normative Appendix) Decolorization Test---Physical Method 5
Appendix C (Normative Appendix) Decolorization Chemistry Test Method---Visual Colorimetric Method 6
Foreword
All technical content in this section is mandatory.
YY 0286 "Special Infusion Set" consists of 6 parts.
--- Part 1. Single-use precision filtration infusion set;
--- Part 2. Single-use burette infusion set;
--- Part 3. One-time use of light infusion sets;
--- Part 4. Infusion sets for single use pressure infusion equipment;
---Part 5. Disposable bottle and bag infusion sets;
--- Part 6. Single-use flow setting fine-tuning infusion set.
This part is the third part of YY 0286.
This part is drafted in accordance with the rules given in GB/T 1.1-2009.
This part is based on GB 18458.3-2005 "Special Infusion Part 3. Disposable Light Insufflator"
Compared with GB 18458.3-2005, the main technical changes except editorial changes are as follows.
--- Modified the introduction;
--- Removed the tag request;
--- Appendix C extraction solution increased 65% ethanol (CH3CH2O) aqueous solution and 50% polyethylene glycol 400 aqueous solution.
Please note that some of the contents of this document may involve patents. The issuing organization of this document is not responsible for identifying these patents.
This part is under the jurisdiction of the National Technical Committee for Standardization of Medical Infusion Devices (SAC/TC106).
This section drafted by. Shandong Province Medical Device Product Quality Inspection Center, Wuhan Zhixun Chuangyuan Technology Development Co., Ltd., Shandong
Xinhua Ande Medical Products Co., Ltd., Jiangxi Hongda Medical Devices Co., Ltd., Tianjin Hana Good Medical Materials Co., Ltd., Beijing Volt Technology
Co., Ltd., Shandong Weigao Group Medical Polymer Products Co., Ltd., Klinnik Medical Devices (Nanchang) Co., Ltd., Jiangxi Sanxin
Medical Technology Co., Ltd.
The main drafters of this section. Luo Hongyu, Luo Yong, Liu Ye, Tian Xiaolei, Wang Tongchao, Wang Haiyin, Chen Yong, Wang Jianfeng, Xia Xinrui, Yan Bo,
Zheng Jinlu.
introduction
With the continuous development of infusion technology and the increasing clinical requirements, some infusions that can adapt to special clinical requirements have been produced.
Device. Since the development of the product is endless, it is not expected to include all special requirements infusion sets in one standard.
Therefore, each part of YY 0286 regulates these dedicated infusion sets only for one clinical special requirement. Some special infusion sets may be combined
Belongs to a variety of special infusion sets, the same part of YY 0286 should be used at the same time.
Some drugs in the clinic need to be infused in the dark, such as sodium nitroprusside, nitroglycerin, vitamin B2, etc., in line with GB 8368
The infusion set does not meet this infusion requirement and therefore requires the use of a dark-protected infusion set as specified in this section of YY 0826.
The parts that are in contact with the liquid insufflator need to have light-proof performance. This part only specifies the light-proof property of the dripper and the pipeline part.
Yes, other components are limited by their external dimensions, so they are not protected from light and are controlled by the manufacturer.
It is the responsibility of the device manufacturer to keep the light insufflator stable during the shelf life and not to decolorize. Standard Appendix B and Appendix C
The decolorization evaluation method of the light insufflator is given. The four alternative solvents given in Appendix C are more suitable for the device manufacturer to carry out the bleaching test.
Applicable incompatible drugs should be identified on the device packaging.
The currently seen infusion sets are protected from light by.
--- A single layer structure made of light-proof pellets;
--- A composite structure made of a light-proof layer and a non-light-proof layer;
--- Partially use the shading device (such as the dripper with a hood) to block;
--- A combination of the above.
When the light-shielding device is equipped with a light-shielding device, the light-shielding test of this standard is not applicable to the light-shielded part.
For infusion sets made of a combination of a light-proof layer and a non-light-proof layer, it is advisable to consider the wall thickness, total wall thickness and joint reliability between layers.
The impact of product use performance.
At the request of the competent national authority, the manufacturer or supplier of the dark-protected infusion set shall submit all materials, material compositions and their
Details of the production method and detailed production information of the dark-proof infusion set, including the chemical name and content of any additives, these additives are
What is added to the raw materials from the light infusion set is also the details of all the used additives.
Special infusion set
Part 3. Disposable light infusion sets
1 Scope
This part of YY 0286 specifies the requirements for single-use, gravity infusion-type infusion sets with liquid-shielding materials added to the light-shielding agent.
Called "light infusion device".
This section also provides guidance on the performance and quality specifications of materials used in the dark.
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article.
Pieces. For undated references, the latest edition (including all amendments) applies to this document.
GB/T 601-2002 Preparation of chemical reagent standard titration solution
GB 8368 Disposable Infusion Set Gravity Infusion
3 General requirements
The requirements specified in GB 8368 apply to this document.
4 materials
The materials for the production of light-proof infusion sets and their components shall meet the requirements of Chapter 5, and the materials in contact with the liquid infusion inhaler shall be
Meet the requirements of Chapters 6 and 7.
5 Physical requirements
5.1 General
The physical requirements of the light insufflator should meet the requirements of GB 8368.
5.2 Light protection
When tested according to Appendix A, the light transmittance of the dark-protected infusion set in the wavelength range of 290 nm to 450 nm shall comply with Table 1.
5.3 Decolorization
5.3.1 When tested in accordance with Appendix B, the dark infusion set should not be discolored.
5.3.2 When tested in accordance with Appendix C, the color of the extract from the dark infusion set should not be darker than the blank.
6 Chemical requirements
Should meet the requirements of GB 8368.
Table 1 Light transmittance limit of each component
Component transmittance /%
Dropper ≤35
Pipeline ≤15
7 Biological requirements
Should meet the requirements of GB 8368.
8 signs
Should meet the requirements of GB 8368.
Light-proof infusion sets should also be marked with light-proof indicators.
9 packaging
Should meet the requirements of GB 8368.
10 type inspection
The provisions of GB 8368 apply.
Appendix A
(normative appendix)
Determination of light transmittance
A.1 Principle
Apply a spectrophotometer with appropriate sensitivity and precision for measuring the light transmission properties of transparent or translucent plastic materials. Correct
For clear plastics, use a spectrophotometer with sensitivity and precision for measuring and recording the amount of light. for translucent plastics, use
The spectrophotometer should also be capable of measuring scattered and parallel light. Use air as a reference to measure the wavelength range from 290nm to 450nm
The transmittance of light.
A.2 Instrument
A.2.1 Spectrophotometer can scan from 290nm to 450nm.
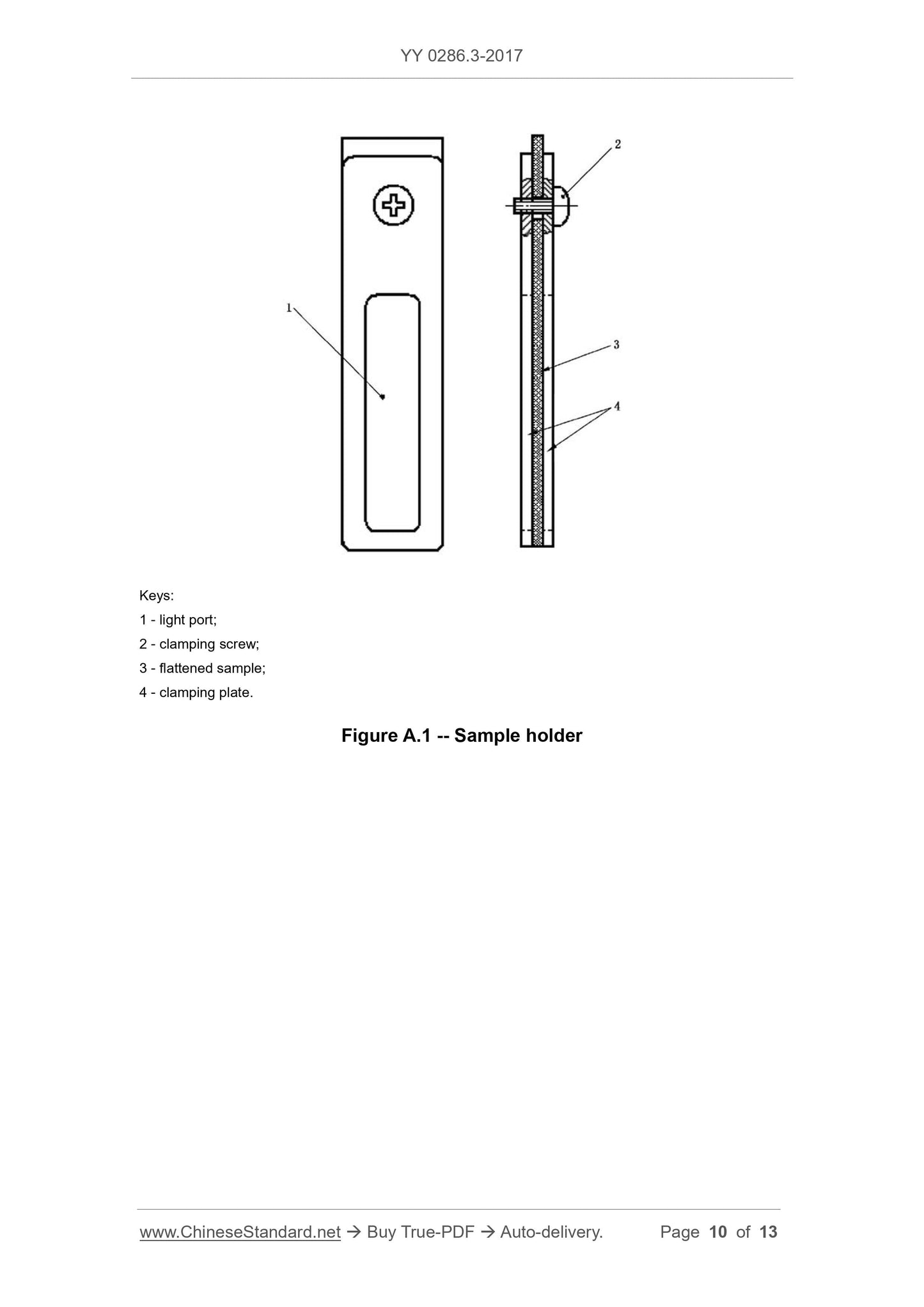
A.2.2 Sample holder, as shown in Figure A.1. The surface is flat and burr-free, and the dimensions are in a cuvette that can be placed vertically into the spectrophotometer.
It is appropriate.
A.3 Sample preparation
Use a suitable tool to cut the dripper and tubing of the dark infusion set separately. Choose a representative average wall thickness and trim it into a suitable piece
Shaped for mounting in the sample holder. After the cutting is completed, clean and dry to avoid surface scratches. If the sample is too small to cover the sample
For the optical port of the product holder, fold the uncovered portion with a suitable paper or tape. The length of the sample must be greater than the length of the slit. The sample is placed in the holder
After that, gently wipe it with lens paper to avoid fingerprints or other smudges where the light passes.
A.4 Test procedure
Place the holder with the sample on the spectrophotometer (concave surface towards the light source) so that the column axis of the sample is parallel to the slit and placed in the slit as much as possible
The heart should be such that the placed sample beam can pass through the surface of the test piece normally and minimize beam reflection loss.
Using air as a reference, the transmittance of the test piece at a continuous wavelength in the range of 290 nm to 450 nm was measured.
Description.
1---optical port;
2---clamping screw;
3---flattened sample;
4---Clamping plate.
Figure A.1 Sample Holder
Appendix B
(normative appendix)
Decolorization test---physical method
B.1 Principle
Put the light-proof infusion set into a high temperature to speed up the color deposition rate, wipe it with absorbent cotton, and observe the discoloration condition.
B.2 Test method
B.2.1 Place the light-proof infusion set in a (60±5) °C incubator for 24 hours. After removal, keep the infusion set clean and let it stand at room temperature for use.
B.2.2 Unfold the light-proof infusion set, take a piece of absorbent cotton (about 0.2g) to wrap a liquid path of the infusion set, and pinch the test part with your finger.
The inner wall of the pipeline is in contact, pulling the absorbent cotton so that the stroke on the liquid path is 1m (including the drip bucket), and then pulling in the opposite direction, so that the stroke is also
1m, open the absorbent cotton to observe, no obvious staining on the absorbent cotton was judged as "no discoloration".
Appendix C
(normative appendix)
Decolorization Chemistry Test Method---Visual Colorimetry
C.1 General
Decolorization Absorbance Test - Chemical method Four different solutions were selected for a 2 h cycle test.
C.2 Preparation of solution
C.2.1 c[HCL]=0.1mol/L hydrochloric acid solution. 1000mL according to the provisions of 4.2 of GB 601-2002;
C.2.2 c[NaOH]=0.1mol/L sodium hydroxide solution. Weigh 4.000g sodium hydroxide and dilute to 1000mL with water.
C.2.3 65% ethanol (CH3CH2O) aqueous solution. Measure 650 mL of absolute ethanol and dilute to 1000 mL with water.
C.2.4 50% Polyethylene Glycol 400 Aqueous Solution. Measure 500 mL of polyethylene glycol 400 and dilute to 1000 mL with water.
C.3 Preparation of extract and blank
Three sets of sterilized dark infusion setters and a 300 mL borosilicate glass flask were connected into a closed loop system. The flask is placed in plus
The temperature of the liquid in the flask was maintained at (37 ± 1) °C on the heater, and 250 mL of a leaching solution given in C.2 was added to 1 L/h.
Speed cycle 2h. For example, a peristaltic pump is applied to a short length of silicone rubber tube, and all the liquid is collected and cooled to obtain a leach solution.
The same leaching solution was used, and the blank solution was prepared in the same manner as the infusion set.
C.4 Test procedure
Take 50 mL of the extract in the corresponding Nessler colorimetric tube, take another 50 mL Nessler colorimetric tube, and add 50 mL of the corresponding blank solution.
Observe the color from the top under a white background.
C.5 result representation
If the color of the extract is darker than the blank, the decolorization test failed.
Get Quotation: Click YY 0286.3-2017 (Self-service in 1-minute)
Historical versions (Master-website): YY 0286.3-2017
Preview True-PDF (Reload/Scroll-down if blank)
YY 0286.3-2017: Special infusion sets - Part 3: Light-resistant infusion sets for single use
YY 0286.3-2017
Special infusion sets -- Part 3. Light-resistant infusion sets for single use
ICS 11.040.20
C31
People's Republic of China Pharmaceutical Industry Standard
Special infusion set
Part 3. Disposable light infusion sets
Part 3. Light-resistantinfusionsetsforsingleuse
Released on.2017-07-17
2019-01-01 implementation
State Food and Drug Administration issued
Content
Foreword I
Introduction II
1 range 1
2 Normative references 1
3 General requirements 1
4 material 1
5 Physical requirements 1
6 Chemical requirements 1
7 Biological requirements 2
8 logo 2
9 Pack 2
10 type test 2
Appendix A (Normative Appendix) Determination of Transmittance 3
Appendix B (Normative Appendix) Decolorization Test---Physical Method 5
Appendix C (Normative Appendix) Decolorization Chemistry Test Method---Visual Colorimetric Method 6
Foreword
All technical content in this section is mandatory.
YY 0286 "Special Infusion Set" consists of 6 parts.
--- Part 1. Single-use precision filtration infusion set;
--- Part 2. Single-use burette infusion set;
--- Part 3. One-time use of light infusion sets;
--- Part 4. Infusion sets for single use pressure infusion equipment;
---Part 5. Disposable bottle and bag infusion sets;
--- Part 6. Single-use flow setting fine-tuning infusion set.
This part is the third part of YY 0286.
This part is drafted in accordance with the rules given in GB/T 1.1-2009.
This part is based on GB 18458.3-2005 "Special Infusion Part 3. Disposable Light Insufflator"
Compared with GB 18458.3-2005, the main technical changes except editorial changes are as follows.
--- Modified the introduction;
--- Removed the tag request;
--- Appendix C extraction solution increased 65% ethanol (CH3CH2O) aqueous solution and 50% polyethylene glycol 400 aqueous solution.
Please note that some of the contents of this document may involve patents. The issuing organization of this document is not responsible for identifying these patents.
This part is under the jurisdiction of the National Technical Committee for Standardization of Medical Infusion Devices (SAC/TC106).
This section drafted by. Shandong Province Medical Device Product Quality Inspection Center, Wuhan Zhixun Chuangyuan Technology Development Co., Ltd., Shandong
Xinhua Ande Medical Products Co., Ltd., Jiangxi Hongda Medical Devices Co., Ltd., Tianjin Hana Good Medical Materials Co., Ltd., Beijing Volt Technology
Co., Ltd., Shandong Weigao Group Medical Polymer Products Co., Ltd., Klinnik Medical Devices (Nanchang) Co., Ltd., Jiangxi Sanxin
Medical Technology Co., Ltd.
The main drafters of this section. Luo Hongyu, Luo Yong, Liu Ye, Tian Xiaolei, Wang Tongchao, Wang Haiyin, Chen Yong, Wang Jianfeng, Xia Xinrui, Yan Bo,
Zheng Jinlu.
introduction
With the continuous development of infusion technology and the increasing clinical requirements, some infusions that can adapt to special clinical requirements have been produced.
Device. Since the development of the product is endless, it is not expected to include all special requirements infusion sets in one standard.
Therefore, each part of YY 0286 regulates these dedicated infusion sets only for one clinical special requirement. Some special infusion sets may be combined
Belongs to a variety of special infusion sets, the same part of YY 0286 should be used at the same time.
Some drugs in the clinic need to be infused in the dark, such as sodium nitroprusside, nitroglycerin, vitamin B2, etc., in line with GB 8368
The infusion set does not meet this infusion requirement and therefore requires the use of a dark-protected infusion set as specified in this section of YY 0826.
The parts that are in contact with the liquid insufflator need to have light-proof performance. This part only specifies the light-proof property of the dripper and the pipeline part.
Yes, other components are limited by their external dimensions, so they are not protected from light and are controlled by the manufacturer.
It is the responsibility of the device manufacturer to keep the light insufflator stable during the shelf life and not to decolorize. Standard Appendix B and Appendix C
The decolorization evaluation method of the light insufflator is given. The four alternative solvents given in Appendix C are more suitable for the device manufacturer to carry out the bleaching test.
Applicable incompatible drugs should be identified on the device packaging.
The currently seen infusion sets are protected from light by.
--- A single layer structure made of light-proof pellets;
--- A composite structure made of a light-proof layer and a non-light-proof layer;
--- Partially use the shading device (such as the dripper with a hood) to block;
--- A combination of the above.
When the light-shielding device is equipped with a light-shielding device, the light-shielding test of this standard is not applicable to the light-shielded part.
For infusion sets made of a combination of a light-proof layer and a non-light-proof layer, it is advisable to consider the wall thickness, total wall thickness and joint reliability between layers.
The impact of product use performance.
At the request of the competent national authority, the manufacturer or supplier of the dark-protected infusion set shall submit all materials, material compositions and their
Details of the production method and detailed production information of the dark-proof infusion set, including the chemical name and content of any additives, these additives are
What is added to the raw materials from the light infusion set is also the details of all the used additives.
Special infusion set
Part 3. Disposable light infusion sets
1 Scope
This part of YY 0286 specifies the requirements for single-use, gravity infusion-type infusion sets with liquid-shielding materials added to the light-shielding agent.
Called "light infusion device".
This section also provides guidance on the performance and quality specifications of materials used in the dark.
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article.
Pieces. For undated references, the latest edition (including all amendments) applies to this document.
GB/T 601-2002 Preparation of chemical reagent standard titration solution
GB 8368 Disposable Infusion Set Gravity Infusion
3 General requirements
The requirements specified in GB 8368 apply to this document.
4 materials
The materials for the production of light-proof infusion sets and their components shall meet the requirements of Chapter 5, and the materials in contact with the liquid infusion inhaler shall be
Meet the requirements of Chapters 6 and 7.
5 Physical requirements
5.1 General
The physical requirements of the light insufflator should meet the requirements of GB 8368.
5.2 Light protection
When tested according to Appendix A, the light transmittance of the dark-protected infusion set in the wavelength range of 290 nm to 450 nm shall comply with Table 1.
5.3 Decolorization
5.3.1 When tested in accordance with Appendix B, the dark infusion set should not be discolored.
5.3.2 When tested in accordance with Appendix C, the color of the extract from the dark infusion set should not be darker than the blank.
6 Chemical requirements
Should meet the requirements of GB 8368.
Table 1 Light transmittance limit of each component
Component transmittance /%
Dropper ≤35
Pipeline ≤15
7 Biological requirements
Should meet the requirements of GB 8368.
8 signs
Should meet the requirements of GB 8368.
Light-proof infusion sets should also be marked with light-proof indicators.
9 packaging
Should meet the requirements of GB 8368.
10 type inspection
The provisions of GB 8368 apply.
Appendix A
(normative appendix)
Determination of light transmittance
A.1 Principle
Apply a spectrophotometer with appropriate sensitivity and precision for measuring the light transmission properties of transparent or translucent plastic materials. Correct
For clear plastics, use a spectrophotometer with sensitivity and precision for measuring and recording the amount of light. for translucent plastics, use
The spectrophotometer should also be capable of measuring scattered and parallel light. Use air as a reference to measure the wavelength range from 290nm to 450nm
The transmittance of light.
A.2 Instrument
A.2.1 Spectrophotometer can scan from 290nm to 450nm.
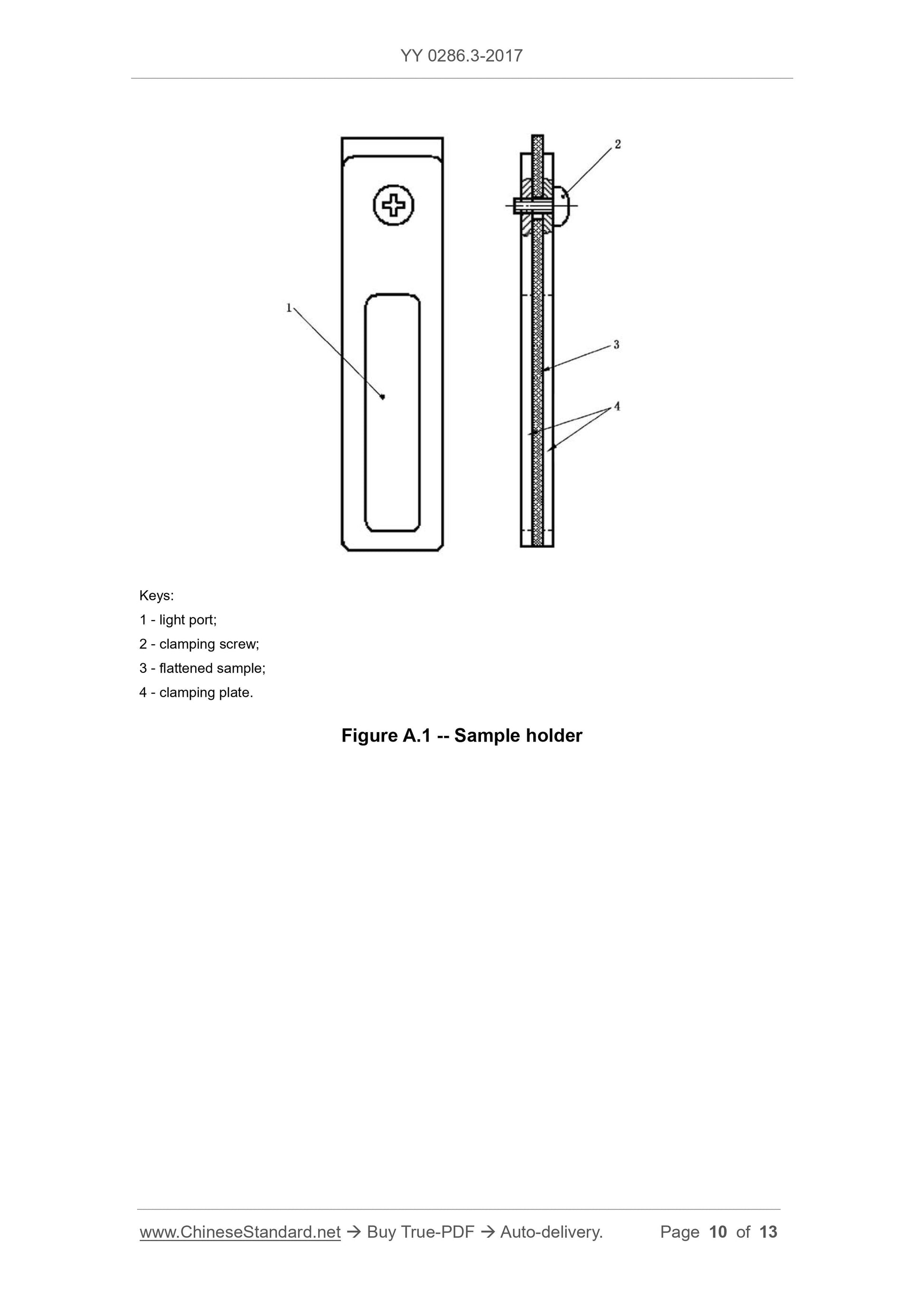
A.2.2 Sample holder, as shown in Figure A.1. The surface is flat and burr-free, and the dimensions are in a cuvette that can be placed vertically into the spectrophotometer.
It is appropriate.
A.3 Sample preparation
Use a suitable tool to cut the dripper and tubing of the dark infusion set separately. Choose a representative average wall thickness and trim it into a suitable piece
Shaped for mounting in the sample holder. After the cutting is completed, clean and dry to avoid surface scratches. If the sample is too small to cover the sample
For the optical port of the product holder, fold the uncovered portion with a suitable paper or tape. The length of the sample must be greater than the length of the slit. The sample is placed in the holder
After that, gently wipe it with lens paper to avoid fingerprints or other smudges where the light passes.
A.4 Test procedure
Place the holder with the sample on the spectrophotometer (concave surface towards the light source) so that the column axis of the sample is parallel to the slit and placed in the slit as much as possible
The heart should be such that the placed sample beam can pass through the surface of the test piece normally and minimize beam reflection loss.
Using air as a reference, the transmittance of the test piece at a continuous wavelength in the range of 290 nm to 450 nm was measured.
Description.
1---optical port;
2---clamping screw;
3---flattened sample;
4---Clamping plate.
Figure A.1 Sample Holder
Appendix B
(normative appendix)
Decolorization test---physical method
B.1 Principle
Put the light-proof infusion set into a high temperature to speed up the color deposition rate, wipe it with absorbent cotton, and observe the discoloration condition.
B.2 Test method
B.2.1 Place the light-proof infusion set in a (60±5) °C incubator for 24 hours. After removal, keep the infusion set clean and let it stand at room temperature for use.
B.2.2 Unfold the light-proof infusion set, take a piece of absorbent cotton (about 0.2g) to wrap a liquid path of the infusion set, and pinch the test part with your finger.
The inner wall of the pipeline is in contact, pulling the absorbent cotton so that the stroke on the liquid path is 1m (including the drip bucket), and then pulling in the opposite direction, so that the stroke is also
1m, open the absorbent cotton to observe, no obvious staining on the absorbent cotton was judged as "no discoloration".
Appendix C
(normative appendix)
Decolorization Chemistry Test Method---Visual Colorimetry
C.1 General
Decolorization Absorbance Test - Chemical method Four different solutions were selected for a 2 h cycle test.
C.2 Preparation of solution
C.2.1 c[HCL]=0.1mol/L hydrochloric acid solution. 1000mL according to the provisions of 4.2 of GB 601-2002;
C.2.2 c[NaOH]=0.1mol/L sodium hydroxide solution. Weigh 4.000g sodium hydroxide and dilute to 1000mL with water.
C.2.3 65% ethanol (CH3CH2O) aqueous solution. Measure 650 mL of absolute ethanol and dilute to 1000 mL with water.
C.2.4 50% Polyethylene Glycol 400 Aqueous Solution. Measure 500 mL of polyethylene glycol 400 and dilute to 1000 mL with water.
C.3 Preparation of extract and blank
Three sets of sterilized dark infusion setters and a 300 mL borosilicate glass flask were connected into a closed loop system. The flask is placed in plus
The temperature of the liquid in the flask was maintained at (37 ± 1) °C on the heater, and 250 mL of a leaching solution given in C.2 was added to 1 L/h.
Speed cycle 2h. For example, a peristaltic pump is applied to a short length of silicone rubber tube, and all the liquid is collected and cooled to obtain a leach solution.
The same leaching solution was used, and the blank solution was prepared in the same manner as the infusion set.
C.4 Test procedure
Take 50 mL of the extract in the corresponding Nessler colorimetric tube, take another 50 mL Nessler colorimetric tube, and add 50 mL of the corresponding blank solution.
Observe the color from the top under a white background.
C.5 result representation
If the color of the extract is darker than the blank, the decolorization test failed.
Share