1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY 0325-2016 English PDF
YY 0325-2016 English PDF
Regular price
$150.00 USD
Regular price
Sale price
$150.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY 0325-2016 (Self-service in 1-minute)
Historical versions (Master-website): YY 0325-2016
Preview True-PDF (Reload/Scroll-down if blank)
YY 0325-2016: Sterile urethral catheter for single use
YY 0325-2016
Sterile urethral catheter for single use
ICS 11.040.20
C31
People's Republic of China Pharmaceutical Industry Standard
Replacing YY 0325-2002
Disposable sterile catheter
Published on.2016-03-23
2018-01-01 implementation
State Food and Drug Administration issued
Foreword
The full technical content of this standard is mandatory.
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
This standard replaces YY 0325-2002 "Single use sterile catheter." Major technical differences compared to YY 0325-2005
as follows.
--- Revised the normative reference document (see Chapter 2);
--- Increased the term nominal balloon volume (see 3.5);
--- "Anti-kink" was modified to "bend resistance" (see 4.7,.2002 edition 4.7);
--- Modified the test method for flow (see 4.8,.2002 edition 4.8);
---Modified parts of symbols and signs (see Chapter 5);
--- Modified the packaging requirements (see Chapter 6).
Please note that some of the contents of this document may involve patents. The issuing organization of this document is not responsible for identifying these patents.
This standard is under the jurisdiction of the National Technical Committee for Standardization of Medical Infusion Devices (SAC/TC106).
This standard is mainly drafted by. Shandong Province Medical Device Product Quality Inspection Center.
Participated in the drafting of this standard. Guangzhou Weili Medical Devices Co., Ltd., Shandong Freda Medical Devices Co., Ltd.
The main drafters of this standard. Wan Min, Song Jin, Wang Yanming, Huang Kaigen.
This standard was first published in January.2002.
Disposable sterile catheter
1 Scope
This standard specifies the terms and definitions, requirements, symbols and signs, packaging, etc. for disposable sterile catheters.
This standard applies to single-use sterilized balloon and balloonless catheters.
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article.
Pieces. For undated references, the latest edition (including all amendments) applies to this document.
GB/T 1962.1 6% (Ruhr) conical joints for syringes, needles and other medical devices - Part 1. General requirements
GB/T 14233.1 Medical infusion, blood transfusion, and injecting machines - Test methods - Part 1
GB/T 15812.1-2005 Non-vascular catheters - Part 1. General performance test methods
GB/T 19633.1 End-sterilized medical device packaging - Part 1. Requirements for materials, sterile barrier systems and packaging systems
YY/T 0313 Requirements for information provided by medical polymer packaging and manufacturers
YY/T 0466.1 Medical devices for use in medical devices - Labels, markings and information provided - Part 1 . General requirements
YY/T 0615.1 Requirements for "sterile" medical devices - Part 1 . Requirements for terminally sterilized medical devices
3 Terms and definitions
The following terms and definitions apply to this document.
3.1
Catheter urethralcatheter
A tubular instrument for the purpose of urinating and flushing the bladder through the urethra into the bladder cavity.
3.2
Balloon volume balooncapacity
The volume of liquid that fills the lumen of the catheter and fills the balloon.
3.3
Body shaft
A portion of the catheter other than the tip, balloon, cone, and/or side hole.
3.4
Outer diameter outsidediameter
The largest dimension measured on the axial vertical section of the pipe.
3.5
Nominal balloon volume nominalbalooncapacity
The nominal volume of the balloon indicated on the tube or on the package.
When the balloon volume is marked in the form of a range, the lower limit is used as the nominal volume.
3.6
Valve valve
A device that controls the flow of liquid.
4 requirements
4.1 General
All tests shall be carried out on products for use.
4.2 Appearance
Catheter for use (eg, as per the manufacturer's instructions), when using normal or corrected visual acuity at 2.5x magnification
When the test is performed, the body, tip, balloon and eyelet should be free of foreign matter and there should be no processing defects or surface defects.
4.3 size
4.3.1 Specification Mark
The urinary catheter uses its nominal outer diameter (mm) to indicate its specification to the nearest 0.1 mm. The tolerance should be ±0.33mm. Balloon volume should
Expressed in milliliters (mL).
Note. Other specification marks can be given at the same time.
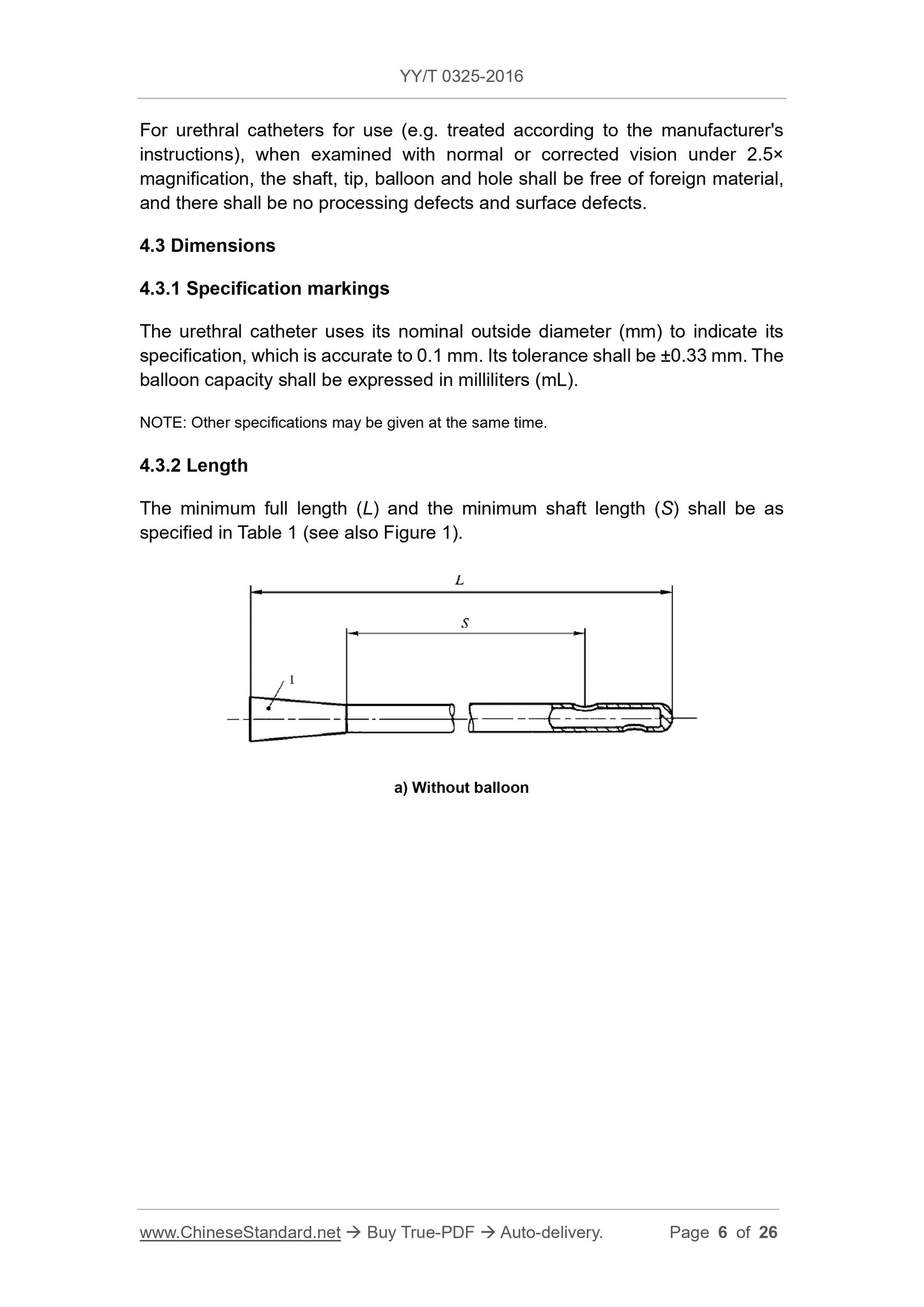
4.3.2 Length
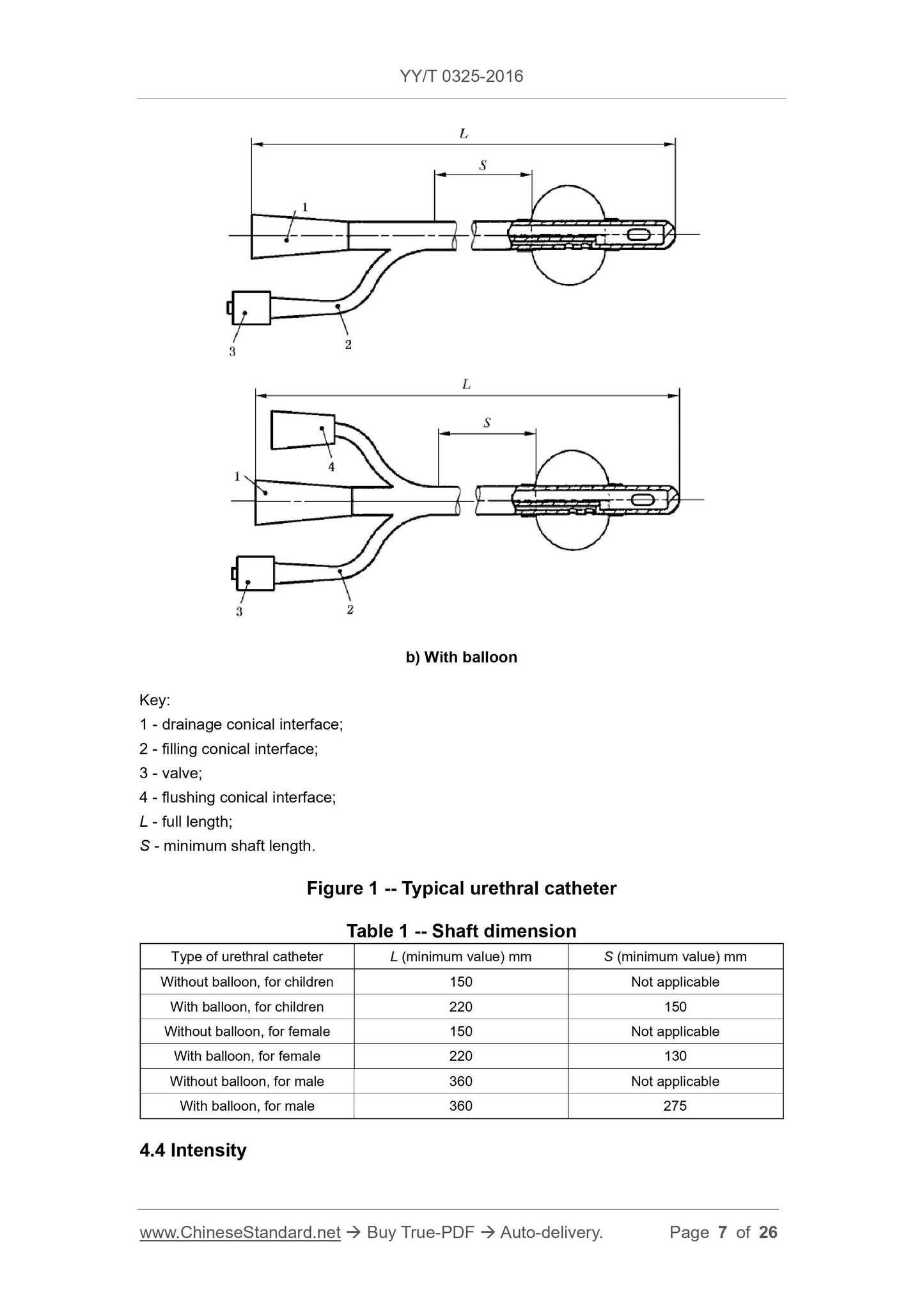
The minimum full length (L) and minimum body length (S) shall be as specified in Table 1 (see also Figure 1).
a) without balloon
Figure 1 Typical catheter
b) has a balloon
Description.
1---Draining tapered interface;
2---fill the tapered interface;
3---valve;
4---washing the tapered interface;
L---full length;
S---minimum body length.
Figure 1 (continued)
Table 1 Body size
Catheter type
L (minimum)
Mm
S (minimum)
Mm
No balloon for children 150 is not applicable
There are balloons for children with 220 150
No balloon female 150 is not applicable
Women with balloons 220 130
No balloon male 360 is not applicable
Have a balloon for men 360 275
4.4 Strength
When tested in accordance with the method given in Appendix A, the tip and tapered interface shall be integral with the body and the body shall be free of breakage.
4.5 Connector separation force
When tested in accordance with the method given in Appendix B, the drain taper interface shall not be separated from the test connector.
4.6 Balloon Reliability
4.6.1 When tested in accordance with the method given in Appendix C, the balloon should be free of leakage and should not affect the drain hole.
Note. If the balloon is not filled, the shape of both ends should be smoothly integrated with the body. At the ambient temperature, the balloon should be filled with water to the specified volume.
Basically bulging.
4.6.2 When tested according to the method given in Appendix D, the water recovery rate shall not be lower than the value specified in Table D.2.
4.7 bending resistance
When tested in accordance with Appendix E, the flow rate of each lumen of the curved catheter should be no less than 50% of the liquid flow of the straight catheter.
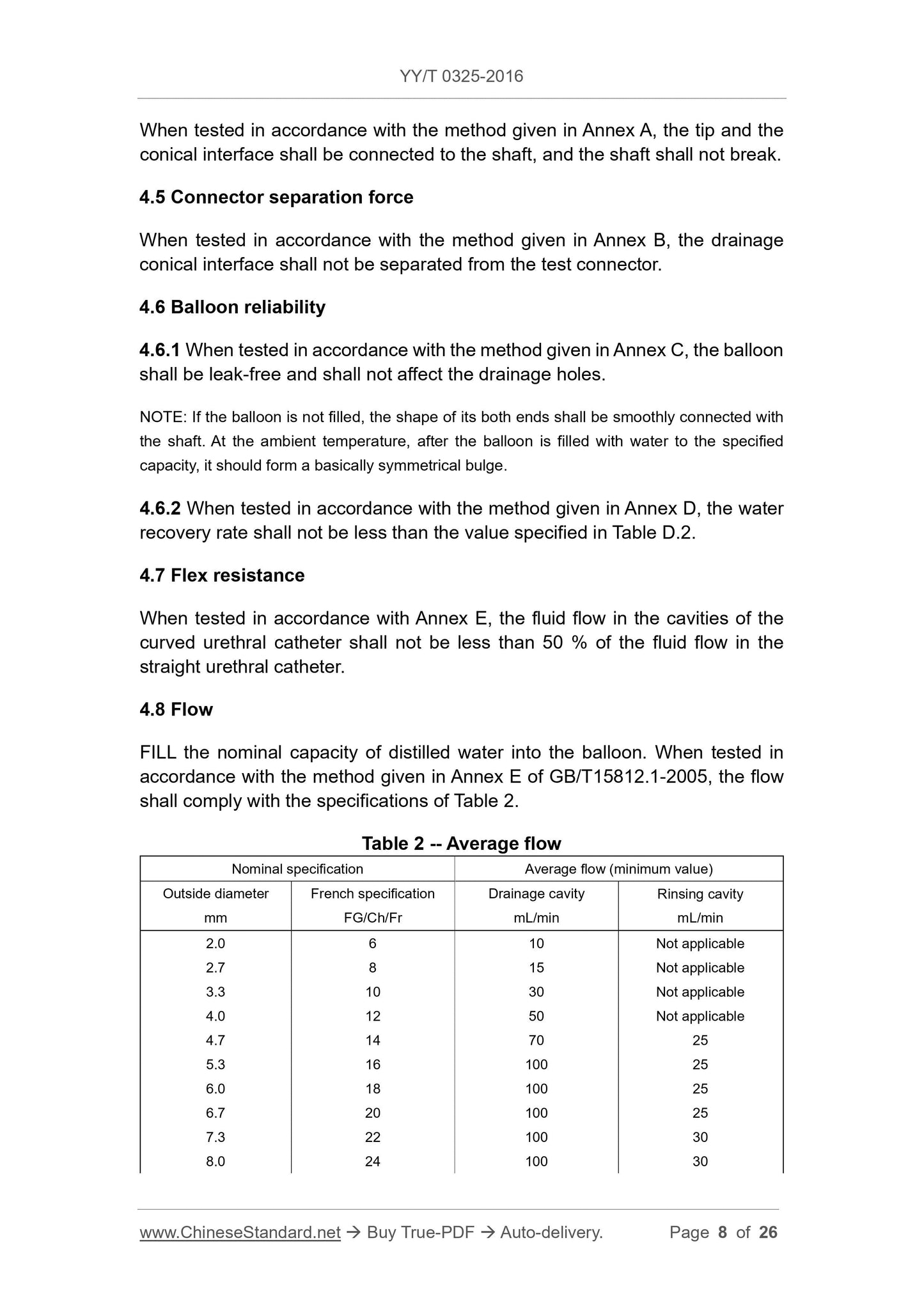
4.8 Traffic
Fill the balloon with the nominal volume of distilled water, according to the method given in Appendix E of GB/T 15812.1-2005, the flow rate should be consistent
Table 2 provides.
Table 2 Average flow
Nominal specification average flow (minimum)
Outer diameter
Mm
French specifications
FG/Ch/Fr
Excretory cavity
mL/min
Flushing chamber
mL/min
2.0
2.7
3.3
4.0
4.7
5.3
6.0
6.7
7.3
8.0
8.7
9.3
10.0
Not applicable
Not applicable
Not applicable
Not applicable
Not applicable
Not applicable
Note. Lubricated catheters have a reduced flow or blockage of the drainage chamber during prolonged use. This standard does not address this risk
Claim.
4.9 Biocompatibility
The catheter should be evaluated for biocompatibility and the results of the evaluation should indicate no biological hazard.
Note. GB/T 16886.1 gives a biocompatibility evaluation method.
4.10 Sterile
The catheter should undergo a confirmed sterilization process to comply with YY/T 0615.1.
Note. The sterility test method is specified in GB/T 14233.2, but this method cannot be used to confirm the sterilization effect of the sterilization batch.
4.11 Corrosion resistance test
If the catheter contains metal components, the specimen shall be free of signs of corrosion when tested in accordance with Appendix A of GB/T 15812.1-2005.
4.12 EO residue
If the catheter is sterilized with ethylene oxide, the residual amount of ethylene oxide should be no more than 10μg/g when tested according to GB/T 14233.1.
5 symbols and signs
5.1 At least the following information should be clearly indicated on the catheter.
a) the nominal balloon volume;
Note. The manufacturer can also indicate the balloon volume in the form of a range.
b) Catheter specification mark (see 4.3.1).
5.2 The symbols or information accompanying the catheter should comply with the requirements of YY/T 0466.1 and YY/T 0313.
6 packaging
6.1 The manufacturer shall be able to provide proof that the package after loading the catheter meets the requirements of GB/T 19633.1.
6.2 If sterilized with ethylene oxide, the packaging should be dialysis material.
6.3 There should be an open trace after the package is opened.
Appendix A
(normative appendix)
Test method for determining the strength of a catheter
A.1 Principle
A balloon catheter may be used in situ for a long time. Therefore, for a balloon catheter, the catheter is immersed in simulated urine before the test.
14d. For a balloonless catheter, this step can be omitted. A pull is applied to the junction between the tip of the catheter and the body. For side
The urinary catheter of the perforation is applied to the perforation; for the catheter without the lateral perforation, a tensile force is applied between the tubular body and the draining conical interface. Unload
After pulling, check the catheter for signs of damage.
A.2 reagent
A.2.1 Simulated urine can be used in any of the following two recipes.
a) Simulated urine consists of the following components (pH approximately 6.6) and the reagents are analytically pure reagents.
Urea 25.0g
Sodium chloride 9.0g
Anhydrous sodium hydrogen phosphate 2.5g
Ammonium chloride 3.0g
Anhydrous potassium dihydrogen phosphate 2.5g
Creatinine 2.0g
Sodium sulfite, anhydrous 3.0g
Distilled water to 1.0L
WARNING. This solution contributes to microbial growth and is likely to be present at the end of the tests described in A.3 and C.3. This
These tests should be carried out by trained personnel. When operating the immersed catheter and discarding contaminated solutions, appropriate
Precaution.
b) The simulated urine consists of the following components (pH about 5.5~7.0) and the reagent is analytically pure reagent.
Sodium chloride 6.17g
Sodium dihydrogen phosphate 4.59g
Sodium citrate 0.944g
Magnesium sulfate 0.463g
Sodium sulfate 2.41g
Potassium chloride 4.75g
Calcium chloride 0.64g
Sodium oxalate 0.043g
Distilled water to 1.0L
Note. The pH is adjusted to be in the range of 5.5 to 7.0 with 1 mol/L ammonium hydroxide (NH4OH) or 1 mol/L ammonium chloride (NH4Cl) solution.
A.3 Instruments
A.3.1 Suspension device with a side hole urinary catheter with a pin that can pass through the urinary catheter drainage hole, the diameter of the pin is in the test catheter
The inner diameter of the excretory cavity is between 50% and 70%. Figure A.1a) shows an example of a suitable device.
For catheters without side perforations, the body is clamped to a suitable clamp.
A.3.2 The connection device of the weight and the drainage chamber, for the catheter with an outer diameter greater than 3.3mm, the total mass of the connecting device and the weight is 1kg;
For a catheter having an outer diameter of less than or equal to 3.3 mm, the total mass of the connecting device and the weight is 0.7 kg.
A.3.3 Water bath, or other device capable of controlling the temperature at (37 ± 2) °C.
A.3.4 Timer.
A.4 steps
The catheter is immersed in the newly prepared simulated urine (A.2.1) and placed in a water bath (A.3.3), controlled at (37 ± 2) °C to allow the balloon and
The body is completely submerged.
The catheter was removed after immersion in simulated urine for 14 days, rinsed with tap water and allowed to dry, and the catheter was placed at (23 ± 2) °C temperature bar.
Under the pieces.
Pass the pin on the suspension device (A.3.1) through the drain hole at the tip of the catheter and hang it (see Figure A.1);
The catheter is suspended by a suitable clamp.
Hold the weight (A.3.2), attach it to the draining conical interface of the catheter, and gently lower the weight until it is free to hang on the catheter.
Hold for 1 min.
Remove the weight and verify the separation and damage of all joints of the catheter and the tearing of the eyelet.
A.5 Test report
The test report should include the following information.
a) identification of the catheter;
b) The connection of the tip to the body and the condition of the eyelet after the test.
Note 1. The diameter D of the pin is 50% of the inner diameter of the drainage lumen of the test catheter.
Between 70%.
a) Suspension and pin examples
Note 2. Size P should be sufficient to allow the pin to tip to the tip of the catheter when loading
The support member should not be brought into contact with the edge of the eyelet.
b) Insert the pin into the catheter hole
Figure A.1 Device and test arrangement for catheter strength test
Description.
1---fill the tapered interface;
2--- draining tapered interface;
3---valve;
4---The direction of the test force.
c) Test arrangement
Figure A.1 (continued)
Appendix B
(normative appendix)
Test method for measuring separation force of excavation cone interface assembly
B.1 Principle
The specified test connector is connected to the draining conical interface of the catheter, and an axial pulling force is applied to check whether the joint is separated.
B.2 Instruments
B.2.1 Test connector, manufactured of rigid material, the dimensions of which are shown in Figure B.1a).
B.2.2 A clamp or similar device for suspending the catheter.
B.2.3 The connection device of the weight test connector, for the catheter with the specification less than or equal to 3.3mm, the total quality of the connection device and the weight
The amount is 0.75kg; for a catheter with a size greater than 3.3mm, the total mass of the connecting device and the weight is 1kg.
B.2.4 Timer.
B.3 steps
The test was carried out at (23 ± 2) °C.
The draining taper interface and connector (B.2.1) of the catheter are cleaned and dried.
Insert the connector into the drain cone interface to a depth of 10 mm or more (ie, to meet or exceed the marking on the connector).
Pi...
Get Quotation: Click YY 0325-2016 (Self-service in 1-minute)
Historical versions (Master-website): YY 0325-2016
Preview True-PDF (Reload/Scroll-down if blank)
YY 0325-2016: Sterile urethral catheter for single use
YY 0325-2016
Sterile urethral catheter for single use
ICS 11.040.20
C31
People's Republic of China Pharmaceutical Industry Standard
Replacing YY 0325-2002
Disposable sterile catheter
Published on.2016-03-23
2018-01-01 implementation
State Food and Drug Administration issued
Foreword
The full technical content of this standard is mandatory.
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
This standard replaces YY 0325-2002 "Single use sterile catheter." Major technical differences compared to YY 0325-2005
as follows.
--- Revised the normative reference document (see Chapter 2);
--- Increased the term nominal balloon volume (see 3.5);
--- "Anti-kink" was modified to "bend resistance" (see 4.7,.2002 edition 4.7);
--- Modified the test method for flow (see 4.8,.2002 edition 4.8);
---Modified parts of symbols and signs (see Chapter 5);
--- Modified the packaging requirements (see Chapter 6).
Please note that some of the contents of this document may involve patents. The issuing organization of this document is not responsible for identifying these patents.
This standard is under the jurisdiction of the National Technical Committee for Standardization of Medical Infusion Devices (SAC/TC106).
This standard is mainly drafted by. Shandong Province Medical Device Product Quality Inspection Center.
Participated in the drafting of this standard. Guangzhou Weili Medical Devices Co., Ltd., Shandong Freda Medical Devices Co., Ltd.
The main drafters of this standard. Wan Min, Song Jin, Wang Yanming, Huang Kaigen.
This standard was first published in January.2002.
Disposable sterile catheter
1 Scope
This standard specifies the terms and definitions, requirements, symbols and signs, packaging, etc. for disposable sterile catheters.
This standard applies to single-use sterilized balloon and balloonless catheters.
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article.
Pieces. For undated references, the latest edition (including all amendments) applies to this document.
GB/T 1962.1 6% (Ruhr) conical joints for syringes, needles and other medical devices - Part 1. General requirements
GB/T 14233.1 Medical infusion, blood transfusion, and injecting machines - Test methods - Part 1
GB/T 15812.1-2005 Non-vascular catheters - Part 1. General performance test methods
GB/T 19633.1 End-sterilized medical device packaging - Part 1. Requirements for materials, sterile barrier systems and packaging systems
YY/T 0313 Requirements for information provided by medical polymer packaging and manufacturers
YY/T 0466.1 Medical devices for use in medical devices - Labels, markings and information provided - Part 1 . General requirements
YY/T 0615.1 Requirements for "sterile" medical devices - Part 1 . Requirements for terminally sterilized medical devices
3 Terms and definitions
The following terms and definitions apply to this document.
3.1
Catheter urethralcatheter
A tubular instrument for the purpose of urinating and flushing the bladder through the urethra into the bladder cavity.
3.2
Balloon volume balooncapacity
The volume of liquid that fills the lumen of the catheter and fills the balloon.
3.3
Body shaft
A portion of the catheter other than the tip, balloon, cone, and/or side hole.
3.4
Outer diameter outsidediameter
The largest dimension measured on the axial vertical section of the pipe.
3.5
Nominal balloon volume nominalbalooncapacity
The nominal volume of the balloon indicated on the tube or on the package.
When the balloon volume is marked in the form of a range, the lower limit is used as the nominal volume.
3.6
Valve valve
A device that controls the flow of liquid.
4 requirements
4.1 General
All tests shall be carried out on products for use.
4.2 Appearance
Catheter for use (eg, as per the manufacturer's instructions), when using normal or corrected visual acuity at 2.5x magnification
When the test is performed, the body, tip, balloon and eyelet should be free of foreign matter and there should be no processing defects or surface defects.
4.3 size
4.3.1 Specification Mark
The urinary catheter uses its nominal outer diameter (mm) to indicate its specification to the nearest 0.1 mm. The tolerance should be ±0.33mm. Balloon volume should
Expressed in milliliters (mL).
Note. Other specification marks can be given at the same time.
4.3.2 Length
The minimum full length (L) and minimum body length (S) shall be as specified in Table 1 (see also Figure 1).
a) without balloon
Figure 1 Typical catheter
b) has a balloon
Description.
1---Draining tapered interface;
2---fill the tapered interface;
3---valve;
4---washing the tapered interface;
L---full length;
S---minimum body length.
Figure 1 (continued)
Table 1 Body size
Catheter type
L (minimum)
Mm
S (minimum)
Mm
No balloon for children 150 is not applicable
There are balloons for children with 220 150
No balloon female 150 is not applicable
Women with balloons 220 130
No balloon male 360 is not applicable
Have a balloon for men 360 275
4.4 Strength
When tested in accordance with the method given in Appendix A, the tip and tapered interface shall be integral with the body and the body shall be free of breakage.
4.5 Connector separation force
When tested in accordance with the method given in Appendix B, the drain taper interface shall not be separated from the test connector.
4.6 Balloon Reliability
4.6.1 When tested in accordance with the method given in Appendix C, the balloon should be free of leakage and should not affect the drain hole.
Note. If the balloon is not filled, the shape of both ends should be smoothly integrated with the body. At the ambient temperature, the balloon should be filled with water to the specified volume.
Basically bulging.
4.6.2 When tested according to the method given in Appendix D, the water recovery rate shall not be lower than the value specified in Table D.2.
4.7 bending resistance
When tested in accordance with Appendix E, the flow rate of each lumen of the curved catheter should be no less than 50% of the liquid flow of the straight catheter.
4.8 Traffic
Fill the balloon with the nominal volume of distilled water, according to the method given in Appendix E of GB/T 15812.1-2005, the flow rate should be consistent
Table 2 provides.
Table 2 Average flow
Nominal specification average flow (minimum)
Outer diameter
Mm
French specifications
FG/Ch/Fr
Excretory cavity
mL/min
Flushing chamber
mL/min
2.0
2.7
3.3
4.0
4.7
5.3
6.0
6.7
7.3
8.0
8.7
9.3
10.0
Not applicable
Not applicable
Not applicable
Not applicable
Not applicable
Not applicable
Note. Lubricated catheters have a reduced flow or blockage of the drainage chamber during prolonged use. This standard does not address this risk
Claim.
4.9 Biocompatibility
The catheter should be evaluated for biocompatibility and the results of the evaluation should indicate no biological hazard.
Note. GB/T 16886.1 gives a biocompatibility evaluation method.
4.10 Sterile
The catheter should undergo a confirmed sterilization process to comply with YY/T 0615.1.
Note. The sterility test method is specified in GB/T 14233.2, but this method cannot be used to confirm the sterilization effect of the sterilization batch.
4.11 Corrosion resistance test
If the catheter contains metal components, the specimen shall be free of signs of corrosion when tested in accordance with Appendix A of GB/T 15812.1-2005.
4.12 EO residue
If the catheter is sterilized with ethylene oxide, the residual amount of ethylene oxide should be no more than 10μg/g when tested according to GB/T 14233.1.
5 symbols and signs
5.1 At least the following information should be clearly indicated on the catheter.
a) the nominal balloon volume;
Note. The manufacturer can also indicate the balloon volume in the form of a range.
b) Catheter specification mark (see 4.3.1).
5.2 The symbols or information accompanying the catheter should comply with the requirements of YY/T 0466.1 and YY/T 0313.
6 packaging
6.1 The manufacturer shall be able to provide proof that the package after loading the catheter meets the requirements of GB/T 19633.1.
6.2 If sterilized with ethylene oxide, the packaging should be dialysis material.
6.3 There should be an open trace after the package is opened.
Appendix A
(normative appendix)
Test method for determining the strength of a catheter
A.1 Principle
A balloon catheter may be used in situ for a long time. Therefore, for a balloon catheter, the catheter is immersed in simulated urine before the test.
14d. For a balloonless catheter, this step can be omitted. A pull is applied to the junction between the tip of the catheter and the body. For side
The urinary catheter of the perforation is applied to the perforation; for the catheter without the lateral perforation, a tensile force is applied between the tubular body and the draining conical interface. Unload
After pulling, check the catheter for signs of damage.
A.2 reagent
A.2.1 Simulated urine can be used in any of the following two recipes.
a) Simulated urine consists of the following components (pH approximately 6.6) and the reagents are analytically pure reagents.
Urea 25.0g
Sodium chloride 9.0g
Anhydrous sodium hydrogen phosphate 2.5g
Ammonium chloride 3.0g
Anhydrous potassium dihydrogen phosphate 2.5g
Creatinine 2.0g
Sodium sulfite, anhydrous 3.0g
Distilled water to 1.0L
WARNING. This solution contributes to microbial growth and is likely to be present at the end of the tests described in A.3 and C.3. This
These tests should be carried out by trained personnel. When operating the immersed catheter and discarding contaminated solutions, appropriate
Precaution.
b) The simulated urine consists of the following components (pH about 5.5~7.0) and the reagent is analytically pure reagent.
Sodium chloride 6.17g
Sodium dihydrogen phosphate 4.59g
Sodium citrate 0.944g
Magnesium sulfate 0.463g
Sodium sulfate 2.41g
Potassium chloride 4.75g
Calcium chloride 0.64g
Sodium oxalate 0.043g
Distilled water to 1.0L
Note. The pH is adjusted to be in the range of 5.5 to 7.0 with 1 mol/L ammonium hydroxide (NH4OH) or 1 mol/L ammonium chloride (NH4Cl) solution.
A.3 Instruments
A.3.1 Suspension device with a side hole urinary catheter with a pin that can pass through the urinary catheter drainage hole, the diameter of the pin is in the test catheter
The inner diameter of the excretory cavity is between 50% and 70%. Figure A.1a) shows an example of a suitable device.
For catheters without side perforations, the body is clamped to a suitable clamp.
A.3.2 The connection device of the weight and the drainage chamber, for the catheter with an outer diameter greater than 3.3mm, the total mass of the connecting device and the weight is 1kg;
For a catheter having an outer diameter of less than or equal to 3.3 mm, the total mass of the connecting device and the weight is 0.7 kg.
A.3.3 Water bath, or other device capable of controlling the temperature at (37 ± 2) °C.
A.3.4 Timer.
A.4 steps
The catheter is immersed in the newly prepared simulated urine (A.2.1) and placed in a water bath (A.3.3), controlled at (37 ± 2) °C to allow the balloon and
The body is completely submerged.
The catheter was removed after immersion in simulated urine for 14 days, rinsed with tap water and allowed to dry, and the catheter was placed at (23 ± 2) °C temperature bar.
Under the pieces.
Pass the pin on the suspension device (A.3.1) through the drain hole at the tip of the catheter and hang it (see Figure A.1);
The catheter is suspended by a suitable clamp.
Hold the weight (A.3.2), attach it to the draining conical interface of the catheter, and gently lower the weight until it is free to hang on the catheter.
Hold for 1 min.
Remove the weight and verify the separation and damage of all joints of the catheter and the tearing of the eyelet.
A.5 Test report
The test report should include the following information.
a) identification of the catheter;
b) The connection of the tip to the body and the condition of the eyelet after the test.
Note 1. The diameter D of the pin is 50% of the inner diameter of the drainage lumen of the test catheter.
Between 70%.
a) Suspension and pin examples
Note 2. Size P should be sufficient to allow the pin to tip to the tip of the catheter when loading
The support member should not be brought into contact with the edge of the eyelet.
b) Insert the pin into the catheter hole
Figure A.1 Device and test arrangement for catheter strength test
Description.
1---fill the tapered interface;
2--- draining tapered interface;
3---valve;
4---The direction of the test force.
c) Test arrangement
Figure A.1 (continued)
Appendix B
(normative appendix)
Test method for measuring separation force of excavation cone interface assembly
B.1 Principle
The specified test connector is connected to the draining conical interface of the catheter, and an axial pulling force is applied to check whether the joint is separated.
B.2 Instruments
B.2.1 Test connector, manufactured of rigid material, the dimensions of which are shown in Figure B.1a).
B.2.2 A clamp or similar device for suspending the catheter.
B.2.3 The connection device of the weight test connector, for the catheter with the specification less than or equal to 3.3mm, the total quality of the connection device and the weight
The amount is 0.75kg; for a catheter with a size greater than 3.3mm, the total mass of the connecting device and the weight is 1kg.
B.2.4 Timer.
B.3 steps
The test was carried out at (23 ± 2) °C.
The draining taper interface and connector (B.2.1) of the catheter are cleaned and dried.
Insert the connector into the drain cone interface to a depth of 10 mm or more (ie, to meet or exceed the marking on the connector).
Pi...
Share