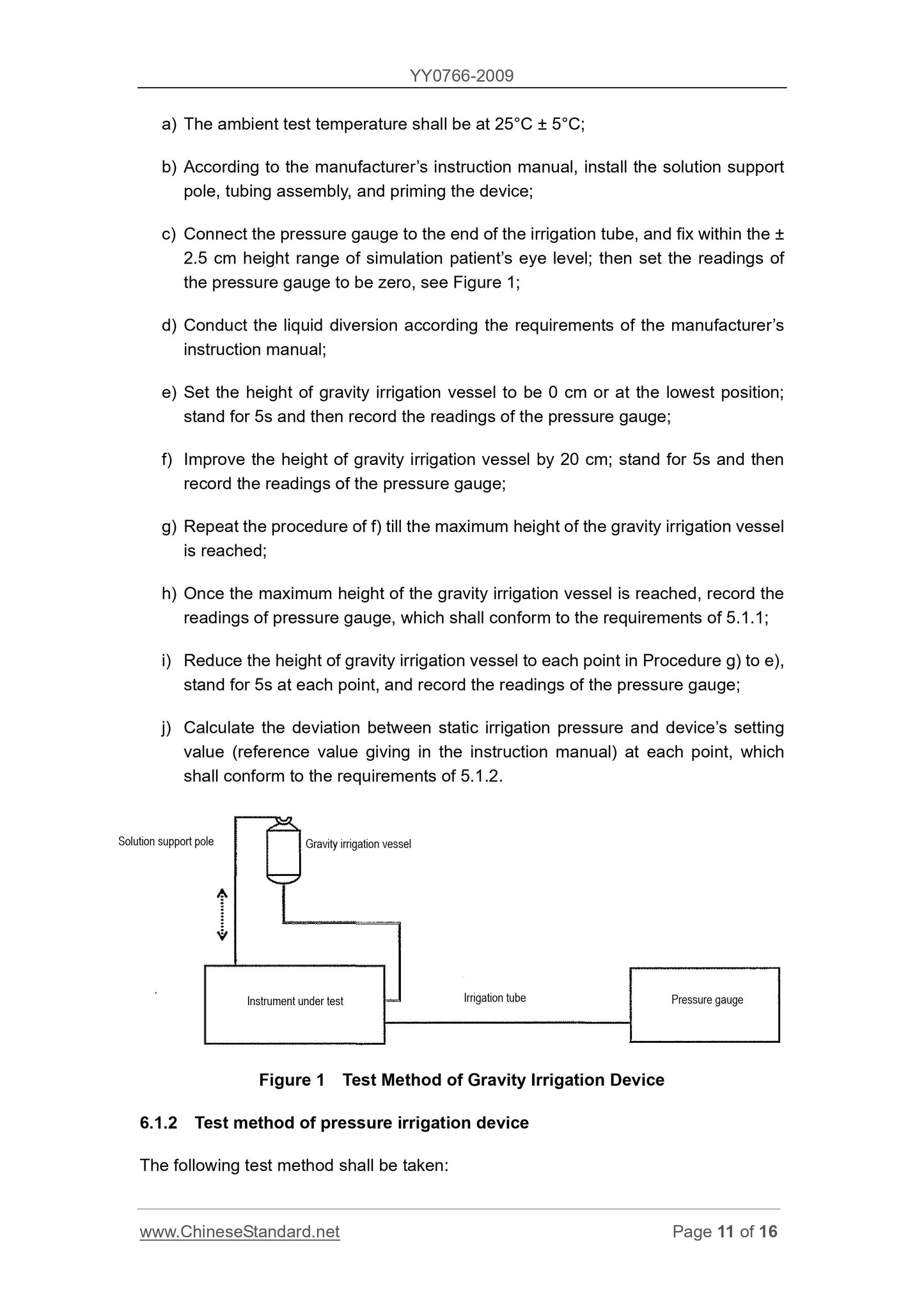
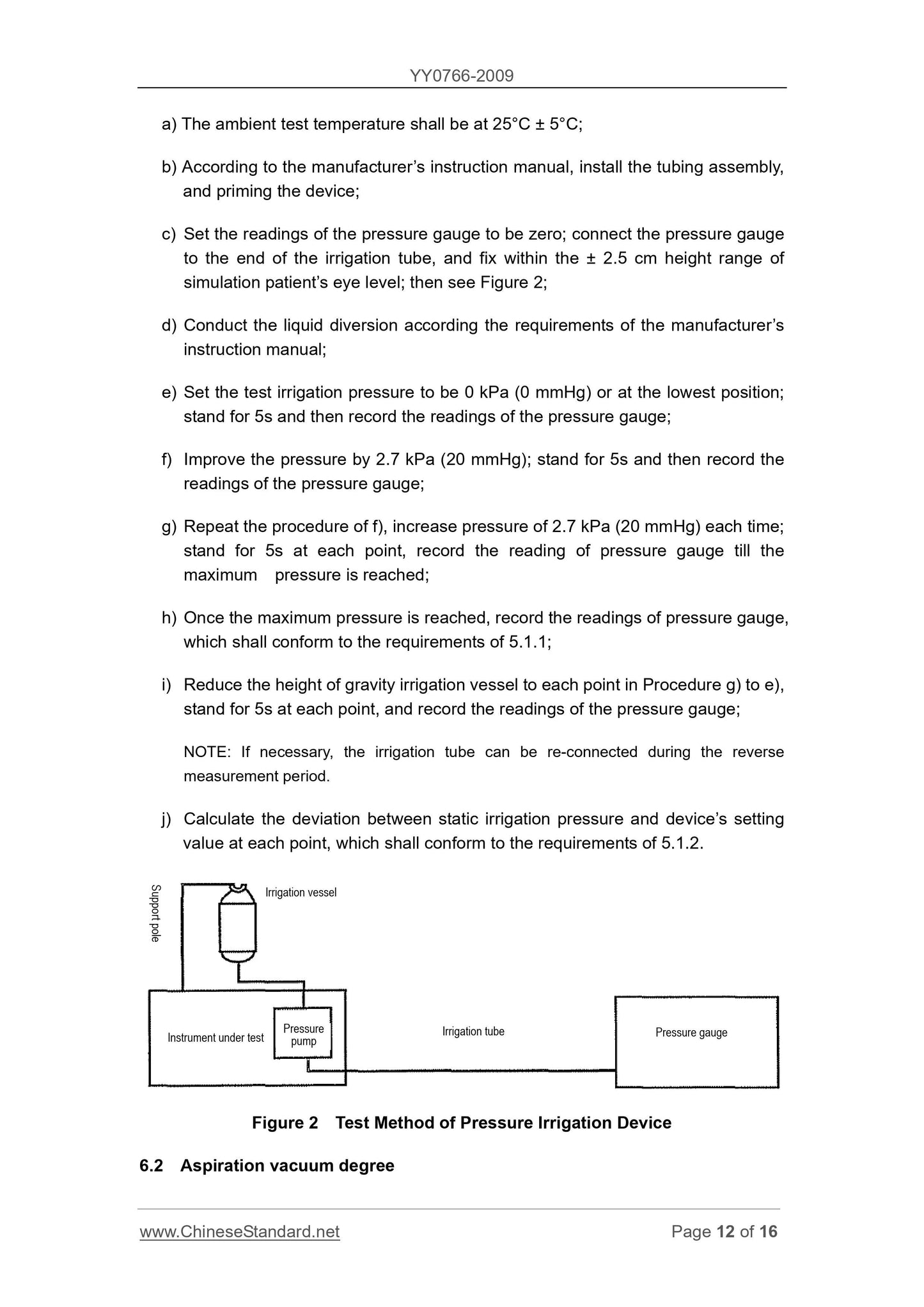
1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY 0766-2009 English PDF
YY 0766-2009 English PDF
Regular price
$140.00 USD
Regular price
Sale price
$140.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY 0766-2009 (Self-service in 1-minute)
Historical versions (Master-website): YY 0766-2009
Preview True-PDF (Reload/Scroll-down if blank)
YY 0766-2009: Lens ultrasonic removal and vitrectomy devices for ophthalmic surgery
Get Quotation: Click YY 0766-2009 (Self-service in 1-minute)
Historical versions (Master-website): YY 0766-2009
Preview True-PDF (Reload/Scroll-down if blank)
YY 0766-2009: Lens ultrasonic removal and vitrectomy devices for ophthalmic surgery
Share