1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY 0789-2010 English PDF
YY 0789-2010 English PDF
Regular price
$155.00 USD
Regular price
Sale price
$155.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Newer version: (Replacing this standard) YY 0789-2024
Get Quotation: Click YY 0789-2010 (Self-service in 1-minute)
Historical versions (Master-website): YY 0789-2024
Preview True-PDF (Reload/Scroll-down if blank)
YY 0789-2010: [Including 2021XG1] Q-Switched Nd: YAG laser ophthalmic system
YY 0789-2010
Q-Switched Nd. YAG laser ophthalmic system
ICS 11.040.70
C41
People's Republic of China Pharmaceutical Industry Standard
Q - switched Nd. YAG laser eye treatment machine
2010-12-27 release
2012-06-01 implementation
State Food and Drug Administration issued
Preface
The implementation of this standard GB 9706.1-2007 "Medical electrical equipment Part 1. General requirements for safety", GB 9706.20-2000 "medical
Electrical equipment - Part 2. Particular requirements for the safety of diagnostic and treatment of laser equipment, GB 7247.1-2001, "Safety of laser products"
Part 1. Equipment Classification, Requirements and User Guide.
This standard is nationalized by the National Standardization Subcommittee for Medical Optics and Instruments (SAC/TC103/SC1).
This standard by the State Food and Drug Administration Hangzhou Medical Device Quality Supervision and Inspection Center drafted.
The main drafters of this standard. Han Jiancheng, Ye Yue Shun, Ye Zhongchen, Huang Dan.
Q - switched Nd. YAG laser eye treatment machine
1 Scope
This standard specifies the basic parameters and product composition of Q-switched Nd. YAG laser ophthalmic treatment machine, technical requirements, test methods and
Logo label, packaging and other requirements.
This standard applies to Q-switched Nd. YAG laser eye treatment machine (hereinafter referred to as treatment machine). Treatment machine through the wavelength of 1064nm
Laser pulse on the human body photolysis/photo-blasting, for the anterior segment of the incision and resection, in order to achieve therapeutic purposes.
2 normative reference documents
The terms of the following documents are hereby incorporated by reference into this standard. Whichever is the date of the reference file, which is followed by all
(Excluding corrigenda) or revisions do not apply to this standard, however, encourage the parties to reach an agreement under this standard
Whether you can use the latest version of these files. For undated references, the latest edition applies to this standard.
GB/T 191-2008 Packaging and storage icon (ISO 780..1997, MOD)
GB 7247.1-2001 Safety of laser products - Part 1. Classification, requirements and user guidance for equipment (idtIEC 60825-1.
1993)
GB 9706.1-2007 Medical electrical equipment - Part 1. General requirements for safety (IEC 60601-1. 1988, IDT)
GB 9706.20-2000 Medical electrical equipment - Part 2. Diagnostic and therapeutic requirements for laser equipment safety
(idtIEC 60601-2-22..1995)
Environmental requirements and test methods for medical appliances GB/T 14710-2009
Ophthalmic instruments - Slit lamp microscopes - YY 0065-2007
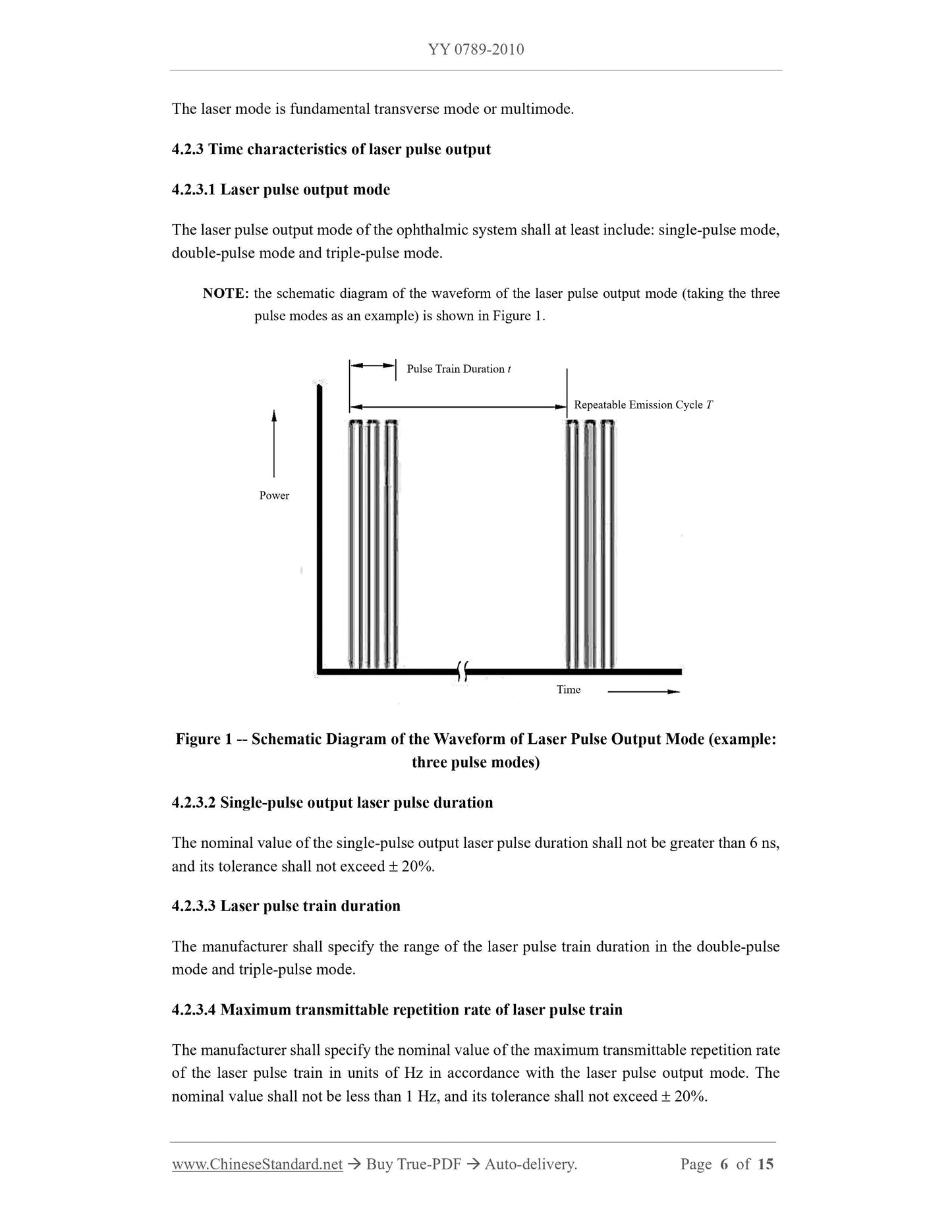
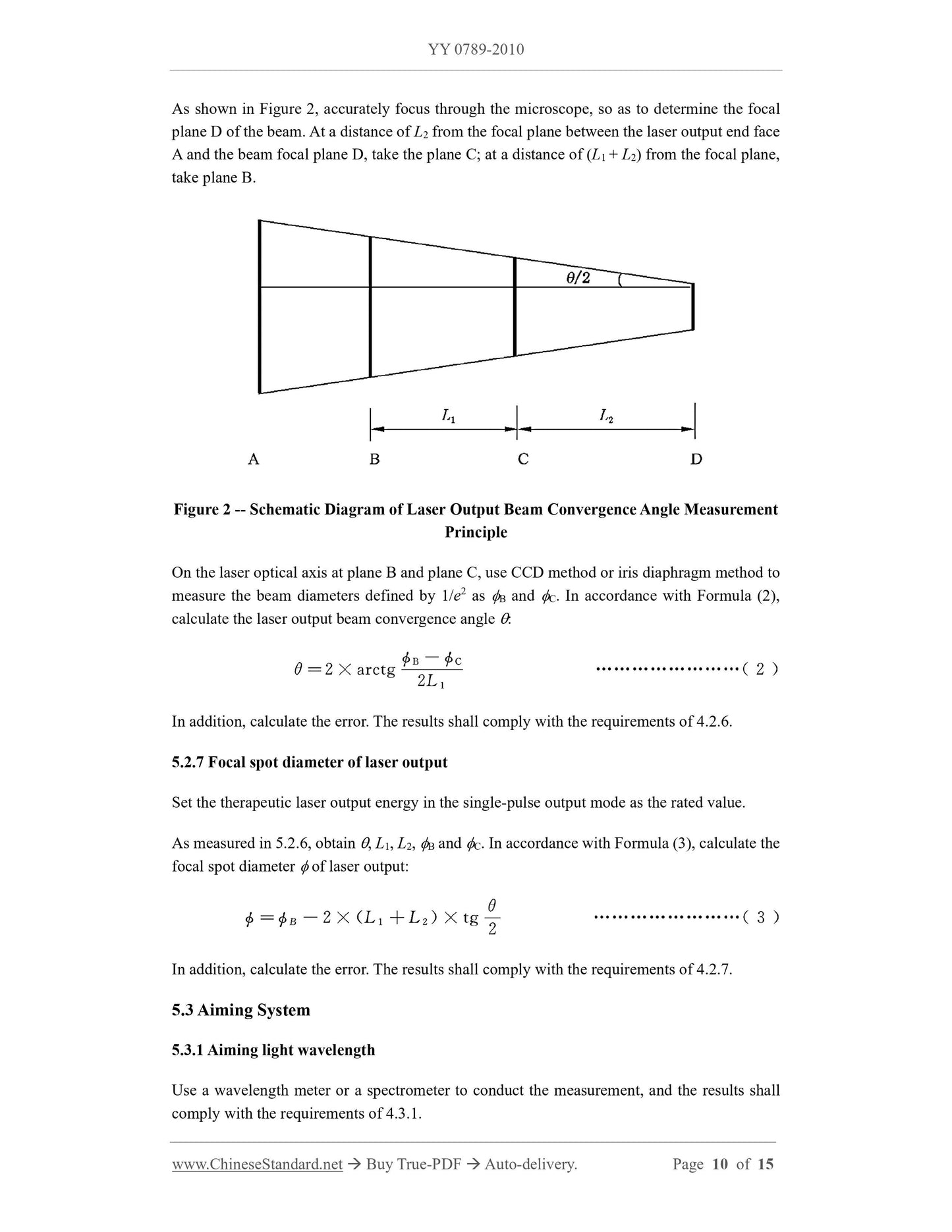
3 product composition and basic parameters
3.1 parts of the treatment machine.
a) Q-switched Nd. YAG lasers;
b) power and control systems;
c) safety and protection systems;
d) slit lamp microscope adapter.
3.2 The basic parameters of the treatment machine.
The manufacturer shall list the following basic parameters in the registered product standard.
a) treatment of laser wavelength;
b) the maximum output energy of the laser pulse/pulse train;
c) treatment of laser pulse duration;
d) treatment of laser output focal spot diameter;
e) aiming at light wavelength;
f) aiming at optical power;
g) Optical parameters of slit lamp microscope adapter.
4 requirements
4.1 normal working conditions
The treatment machine should be able to function properly under the following conditions.
Newer version: (Replacing this standard) YY 0789-2024
Get Quotation: Click YY 0789-2010 (Self-service in 1-minute)
Historical versions (Master-website): YY 0789-2024
Preview True-PDF (Reload/Scroll-down if blank)
YY 0789-2010: [Including 2021XG1] Q-Switched Nd: YAG laser ophthalmic system
YY 0789-2010
Q-Switched Nd. YAG laser ophthalmic system
ICS 11.040.70
C41
People's Republic of China Pharmaceutical Industry Standard
Q - switched Nd. YAG laser eye treatment machine
2010-12-27 release
2012-06-01 implementation
State Food and Drug Administration issued
Preface
The implementation of this standard GB 9706.1-2007 "Medical electrical equipment Part 1. General requirements for safety", GB 9706.20-2000 "medical
Electrical equipment - Part 2. Particular requirements for the safety of diagnostic and treatment of laser equipment, GB 7247.1-2001, "Safety of laser products"
Part 1. Equipment Classification, Requirements and User Guide.
This standard is nationalized by the National Standardization Subcommittee for Medical Optics and Instruments (SAC/TC103/SC1).
This standard by the State Food and Drug Administration Hangzhou Medical Device Quality Supervision and Inspection Center drafted.
The main drafters of this standard. Han Jiancheng, Ye Yue Shun, Ye Zhongchen, Huang Dan.
Q - switched Nd. YAG laser eye treatment machine
1 Scope
This standard specifies the basic parameters and product composition of Q-switched Nd. YAG laser ophthalmic treatment machine, technical requirements, test methods and
Logo label, packaging and other requirements.
This standard applies to Q-switched Nd. YAG laser eye treatment machine (hereinafter referred to as treatment machine). Treatment machine through the wavelength of 1064nm
Laser pulse on the human body photolysis/photo-blasting, for the anterior segment of the incision and resection, in order to achieve therapeutic purposes.
2 normative reference documents
The terms of the following documents are hereby incorporated by reference into this standard. Whichever is the date of the reference file, which is followed by all
(Excluding corrigenda) or revisions do not apply to this standard, however, encourage the parties to reach an agreement under this standard
Whether you can use the latest version of these files. For undated references, the latest edition applies to this standard.
GB/T 191-2008 Packaging and storage icon (ISO 780..1997, MOD)
GB 7247.1-2001 Safety of laser products - Part 1. Classification, requirements and user guidance for equipment (idtIEC 60825-1.
1993)
GB 9706.1-2007 Medical electrical equipment - Part 1. General requirements for safety (IEC 60601-1. 1988, IDT)
GB 9706.20-2000 Medical electrical equipment - Part 2. Diagnostic and therapeutic requirements for laser equipment safety
(idtIEC 60601-2-22..1995)
Environmental requirements and test methods for medical appliances GB/T 14710-2009
Ophthalmic instruments - Slit lamp microscopes - YY 0065-2007
3 product composition and basic parameters
3.1 parts of the treatment machine.
a) Q-switched Nd. YAG lasers;
b) power and control systems;
c) safety and protection systems;
d) slit lamp microscope adapter.
3.2 The basic parameters of the treatment machine.
The manufacturer shall list the following basic parameters in the registered product standard.
a) treatment of laser wavelength;
b) the maximum output energy of the laser pulse/pulse train;
c) treatment of laser pulse duration;
d) treatment of laser output focal spot diameter;
e) aiming at light wavelength;
f) aiming at optical power;
g) Optical parameters of slit lamp microscope adapter.
4 requirements
4.1 normal working conditions
The treatment machine should be able to function properly under the following conditions.
Share