1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY 0844-2011 English PDF
YY 0844-2011 English PDF
Regular price
$150.00 USD
Regular price
Sale price
$150.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY 0844-2011 (Self-service in 1-minute)
Historical versions (Master-website): YY 0844-2011
Preview True-PDF (Reload/Scroll-down if blank)
YY 0844-2011: Laser therapeutic equipment. Pulsed carbon dioxide laser treating instrument
YY 0844-2011
Laser therapeutic equipment.Pulsed carbon dioxide laser treating instrument
ICS 11.040.60
C41
People's Republic of China pharmaceutical industry standards
Laser therapy equipment
Pulsed carbon dioxide laser treatment machine
Issued on. 2011-12-31
2013-06-01 implementation
State Food and Drug Administration issued
Foreword
This standard was drafted in accordance with GB/T 1.1-2009 given rules.
This standard GB 9706.1-2007 "Medical Electrical Equipment Part 1. General requirements for safety", GB 9706.20-2000 "Medical
Electrical equipment - Part 2. diagnostic and therapeutic laser equipment requirements for safety ", GB 7247.1-2001" Safety of Laser Products
Part 1. Equipment Classification, Requirements and User's Guide. "
Please note that some of the content of this document may involve patents. Release mechanism of the present document does not assume responsibility for the identification of these patents.
This standard was proposed by the State Food and Drug Administration.
This standard by the national medical and optical instruments Standardization Technical Committee (SAC/TC103/SC1) centralized.
This standard by the State Food and Drug Administration Hangzhou Medical Device Quality Supervision and Inspection Center, Zhejiang Medical Device Testing, Chongqing
Beijing and Chongqing Laser Technology Co., Ltd. is responsible for drafting.
The main drafters of this standard. Han Jian City, Zhou Zhikang, Du Kun, Ye Yueshun hole Peng.
Laser therapy equipment
Pulsed carbon dioxide laser treatment machine
1 Scope
This standard specifies the pulsed carbon dioxide laser treatment of the basic parameters and the composition of products, technical requirements, test methods, and signs and markings
Sign, packaging, etc., the present standard-setting product standards for the registration of medical devices pulsed carbon dioxide laser treatment machine manufacturer's specifications.
This standard applies to pulsed carbon dioxide laser treatment and containing both continuous wave operation mode contains only pulse operation mode, pulse
Run the carbon dioxide laser treatment machine pulse operation section (hereinafter referred to as the treatment machine). The standard refers to "Pulse", the pulse
Chong duration (pulse width) is less than 0.25s. Treatment machine via a wavelength of 10.6μm pulsed laser vaporization of tissue, carbon
Of freezing and irradiation for therapeutic purposes.
Containing both continuous wave operation mode, pulse operation mode carbon dioxide laser treatment, which should be consistent with the continuous wave operation section
GB 11748, its pulse operation section shall comply with this standard.
2 Normative references
The following documents for the application of this document is essential, dated references, only the dated version suitable for use herein
Member. For undated references, the latest edition (including any amendments) applies to this document.
GB/T 191 Packaging - Pictorial signs
GB 7247.1 Safety of laser products - Part 1. Equipment Classification, Requirements and User's Guide
GB 9706.1 Medical Electrical Equipment Part 1. General requirements for safety
GB 9706.20 Medical electrical equipment - Part 2. diagnostic and therapeutic laser equipment requirements for the safety
GB/T 14710 medical electrical environmental requirements and test methods
GB/T 17736 laser safety goggles main parameters of the test method
YY 91057 medical foot switch General technical conditions
ISO 11146 Test methods for laser and laser-related equipment laser beam widths, divergence angles and beam propagation ratios (Lasersand
Laser-relatedequipmenttestmethodsforlaserbeamwidths, divergenceanglesandbeampropagation
ratios)
3 Terms and Definitions
Terms and definitions defined in GB 7247.1 and the following terms and definitions apply to this document.
3.1
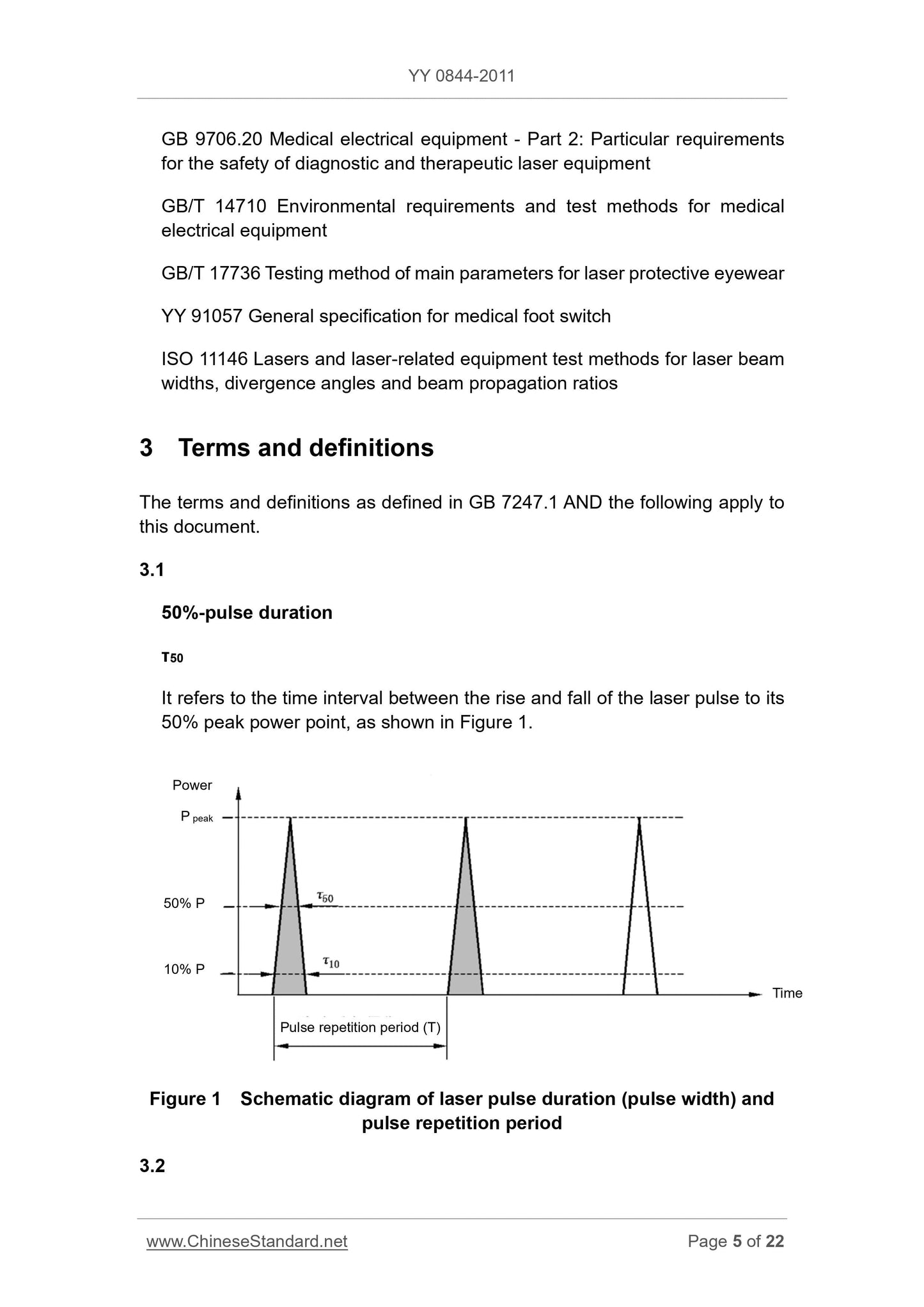
50% of the pulse duration (pulse width of 50%) 50% -pulseduration
τ50
Laser pulse rise time and fall to 50% of its peak power point interval between, see Figure 1.
Figure 1 laser pulse duration (pulse width) and pulse repetition cycle schematic
3.2
10% of the pulse duration (pulse width of 10%) 10% -pulseduration
τ10
Laser pulse rise time and fall to 10% of its peak power point interval between, see Figure 1.
3.3
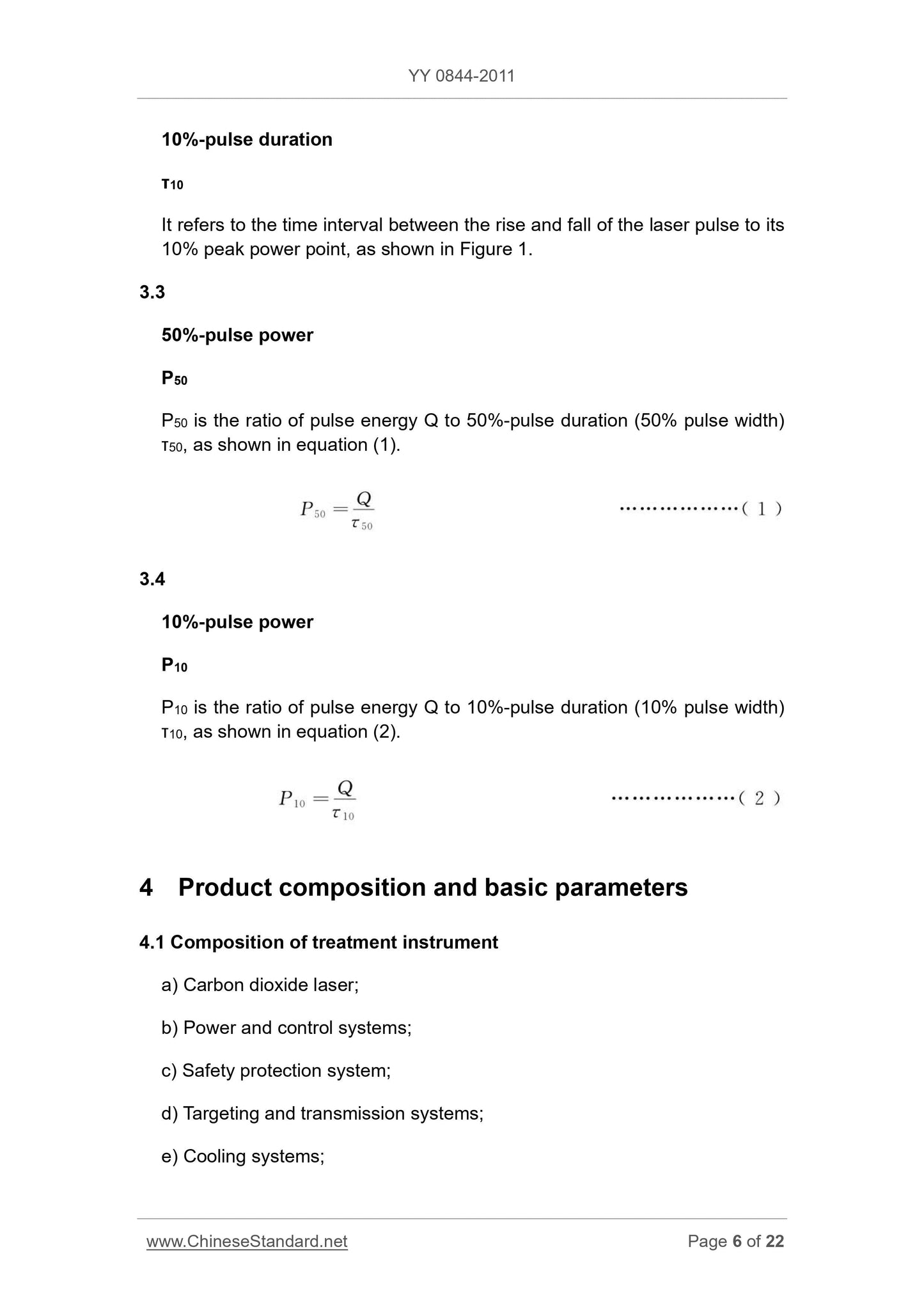
50% 50% -pulsepower pulse power
P50
P50 is a pulse energy Q pulse duration and 50% (50% pulse width) ratio of τ50, see formula (1).
P50 = Qτ50
(1)
3.4
10% 10% -pulsepower pulse power
P10
Q energy pulse P10 with a pulse duration of 10% (10% pulse width) τ10 ratio, see formula (2).
P10 = Qτ10
(2)
4 product composition and basic parameters
4.1 part of the treatment machine
a) carbon dioxide laser;
b) power and control systems;
c) Security;
d) aim, the transmission system;
e) cooling system;
f) output system and accessories (may include an output handpiece, the scanning device/dot output devices, etc.).
4.2 Treatment of basic parameters
a) therapeutic laser wavelength and mode;
b) treatment laser pulse energy Q output terminal, a burst of energy Q series, the average power Pav, pulse power P50 and P10;
c) treatment of the laser pulse duration (pulse width) τ (τ50 and τ10), the pulse repetition period T or the pulse repetition frequency f or accounting
Air ratio of [eta] (see Fig. 1, Fig. 2);
d) for the duration of the laser pulse train (burst width) T1, the burst interval T2 (see FIG. 2) or burst repetition period
T string; string within the number of pulses n, train within the pulse duration (pulse width) τ (τ50 and τ10), the string within the pulse repetition period T (see
Figures 1, 2);
e) Treatment of laser divergence angle of the output terminal, coke (light) spot diameter;
f) aiming light wavelength;
g) aiming light power.
4.3 Safety Category
Manufacturers should be listed in the following categories registered product safety standards.
a) class and type in accordance with the provisions of GB 9706.1;
b) Type of laser radiation in accordance with the provisions of GB 7247.1.
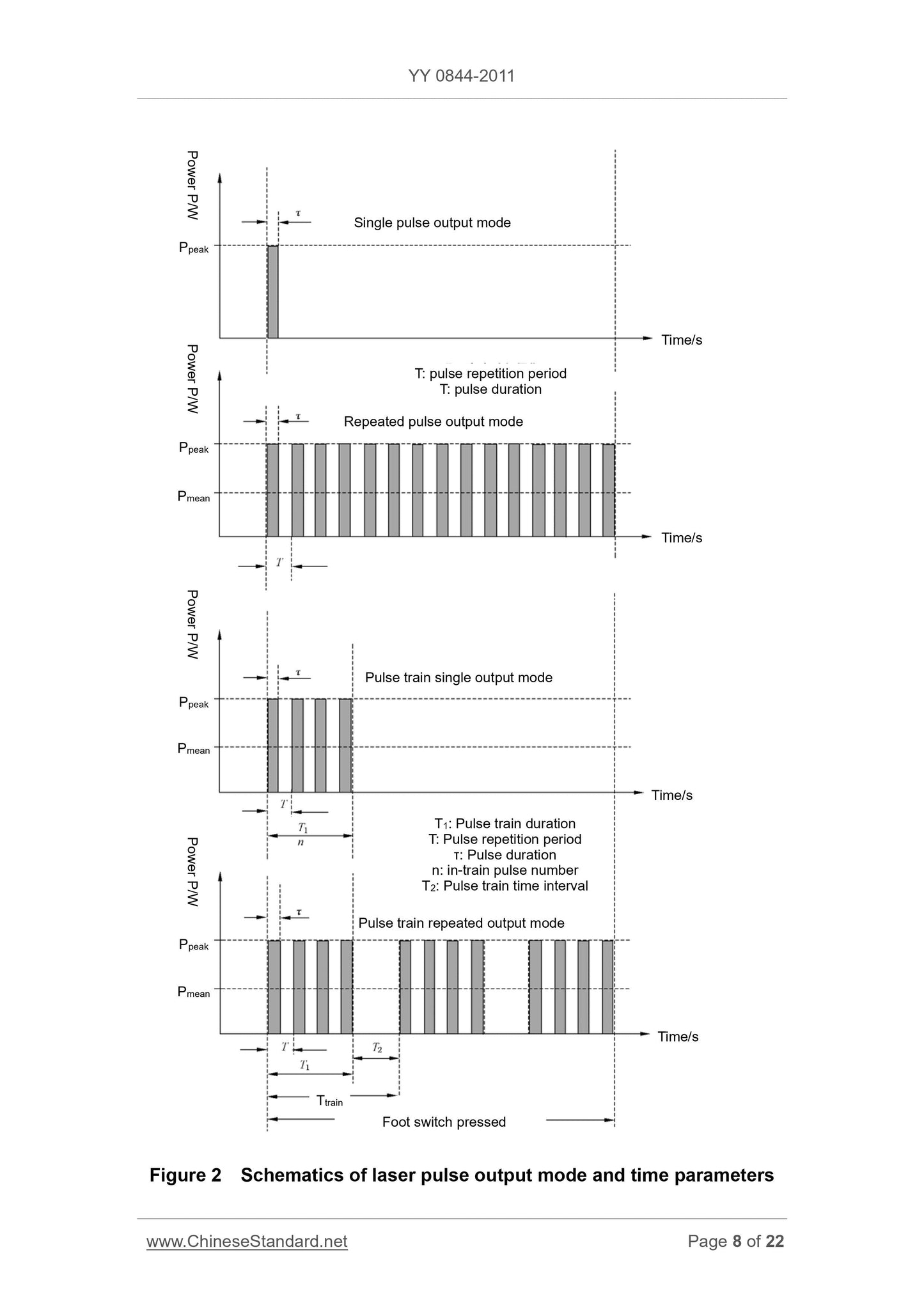
2 laser pulse output and time parameters Schematic diagram
5 Requirements
5.1 Normal operating conditions
Manufacturers should provide at least the following parameters normal working conditions.
--- Ambient temperature;
---Relative humidity;
---using electric.
5.2 Laser Treatment
5.2.1 laser wavelength
10.6μm ± 0.1μm.
5.2.2 laser mode
Fundamental transverse mode or multi-mode.
Its output laser pulse temporal characteristics 5.2.3 (Figure 1, Figure 2)
5.2.3.1 The manufacturer shall specify the laser pulse output may include (see Figure 2).
a) one-shot pulse output mode;
b) pulse repetition output;
c) a single pulse train output;
d) burst repetition output.
5.2.3.2 laser pulse duration (pulse width) τ50 and τ10, pulse repetition period T or the pulse repetition frequency f or the duty ratio η.
a) If the τ50 by the operator can not adjust, the manufacturer should specify the corresponding output τ50 nominal value and tolerances, or scope of τ50;
And gives the corresponding τ10 nominal value and tolerance, or the range τ10. If T is not available by the operator to adjust, the manufacturer shall specify
The corresponding output nominal value and tolerance, or from T T's; above tolerance shall not exceed ± 20%.
Note. tolerances. refers to the measurement of the actual output minus the value of its nominal value, or set its tolerance nominal value or setpoint (deviation of the actual output of the measurement
Algebraic difference value). Following the same.
b) If τ50 adjusted by the operator, the manufacturer should specify the corresponding output setting range and tolerance τ50 at the same time to give the corresponding
The τ10 scope and tolerance; if T or f or η adjustment by the operator, the manufacturer should specify the corresponding output T or f
Or η setting range and tolerance; above tolerance shall not exceed ± 20%.
5.2.3.3 Laser burst duration (burst width) within the string and the Tl pulse number n (see Figure 2).
a) If the T1, n can not be adjusted by the operator, the manufacturer shall specify the nominal T1 nominal value and tolerance (not to exceed ± 20%) and n
Value, or T1, n ranges;
b) If the T1, n adjustment by the operator, the manufacturer should provide T1 set range and tolerance (not to exceed ± 20%) and setting n
range.
5.2.3.4 Laser burst interval T2 (see FIG. 2) or the burst repetition period T string.
a) If the T2, T string can not be adjusted by the operator, the manufacturer should specify the corresponding output T2 or T series of the nominal value and tolerances, or
T2 or scope of T string; above tolerance shall not exceed ± 20%.
b) If T2 or T series adjusted by the operator, the manufacturer should specify the corresponding output T2 or T string setting range and tolerance;
Above tolerance shall not exceed ± 20%.
5.2.4 laser pulse (train) terminal output energy and power
5.2.4.1 pulse energy Q, Q energy burst string.
a) The manufacturer shall specify the corresponding output pulse energy ratings QR, and gives the corresponding τ50 and τ10; manufacturers should be regulated
Given under the corresponding output pulse train energy rating Q series R, and gives the corresponding n; maximum output of the machine should not treat small
In QR and Q series R;
Note. RATINGS nominal amount of the output of a standard middle finger under the specified conditions of use and performance of the normal operation of the maximum output value. Following the same.
b) If the operator Q can be adjusted, the manufacturer shall specify the scope and Q set tolerance, and gives the corresponding τ50 and τ10; if Q series
Adjusted by the operator, the manufacturer shall specify the Q train set range and tolerance, and gives the corresponding n. Above shall not tolerance
More than ± 20%.
5.2.4.2 Terminal output average power Pav, pulse power P50 and the corresponding P10 (Figure 1, Figure 2).
a) The manufacturer shall specify the average power rating PavR, pulse power rating P50R respective output mode, but given the P50R
Corresponding P10R; maximum output of the machine should not be less than the treatment PavR, P50R;
b) If Pav adjusted by the operator, the manufacturer should specify the corresponding output Pav setting range and allow the difference; if P50 can be operated
On regulation, the manufacturer should specify the corresponding output P50 setting range and allow its poor, and gives the corresponding P10; more
Tolerance should not exceed ± 20%.
5.2.5 terminal laser output power instability St
If the treatment machine has a pulse repetition output and (or) repeatedly outputs a burst mode, the manufacturer should provide power instability St, St
Should not exceed ± 10%.
5.2.6 Terminal laser output power/energy reproducibility RP
If the treatment machine has a pulse repetition output and (or) repeatedly outputs a burst mode, the manufacturer shall specify the level of the corresponding output mode
Average power reproducibility RP, RP should not exceed ± 10%;
If the treatment machine has a one-shot pulse output mode and (or) a single pulse train output, manufacturers should provide appropriate energy output under
Reproducibility RP, RP should not exceed ± 10%.
5.2.7 terminal laser divergence angle and coke (light) spot diameter
The manufacturer shall specify the terminal and the divergence angle of the laser output coke (light) spot diameter and the nominal value of tolerance, tolerance should not exceed ± 20%.
5.3 Targeting System
5.3.1 aiming light wavelength
The manufacturer shall specify the nominal value of the wavelength of light to aim and tolerance (tolerance shall not exceed ± 5nm), or wavelength range.
5.3.2 aiming light output terminal
It should be less than 5mW.
5.4 light guide system
5.4.1 The treatment uses a carbon dioxide laser tube length L> when 500mm, shall be equipped with a light guide arm or other light guide system.
5.4.2 light guide system within the scope of their freedom should be no snags and dead phenomenon.
5.5 cooling system using a cooling liquid
5.5.1 liquid-cooled cooling system should be no leakage.
5.5.2 When using liquid cooling liquid cooling system stop, should be able to automatically cut off the laser power supply.
5.6 laser radiation category
The manufacturer shall in accordance with the provisions of GB 7247.1 laser radiation therapy machine category.
5.7 Output Accessories
Treatment machine has an output accessory, the manufacturer shall specify the characteristics of the various parameters of the laser output accessory, including these attachments work output operation area
Range (or nominal) shape parameters and tolerance, tolerance should not exceed ± 20%.
5.8 Appearance
5.8.1 treatment machine should look neat, uniform color, no corrosion, coating peeling, scars, scratches, deformation and other defects.
5.8.2 treatment machine text and markings should be clearly visible.
5.8.3 Treatment control adjustment mechanism should be flexible and reliable, no loosening the fastening parts, buttons, switches feel clear and reliable operation.
5.9 Laser protective glasses
Therapy machine should be configured to meet the following requirements of laser protection glasses.
5.9.1 protective glasses for laser therapy wavelengths of light density. ≥4.
5.9.2 Visible transmittance. ≥65%.
It shall be marked with the wavelength range and optical density protection 5.9.3 on laser protection glasses.
5.10 footswitch
Shall comply with the requirements of 2.9 and 2.5,2.6,2.7,2.8 YY 91057-1999.
5.11 Safety
Therapy machine should be consistent with GB 9706.1, GB 9706.20, GB 7247.1 requirements of.
The manufacturer shall, respectively, in accordance with GB 9706.1, GB 9706.20, GB 7247.1 provisions of its security features (including an insulating panel) and apply
Provisions.
5.12 environmental adaptability
Environmental adaptability treatment machine should be consistent with GB/T 14710 requirements; manufacturers should give a specific test conditions and test items. test
Project should contain at least 5.2.4.2a) laser average output power rating PavR.
6 Test methods
6.1 Test methods listed in this chapter t...
Get Quotation: Click YY 0844-2011 (Self-service in 1-minute)
Historical versions (Master-website): YY 0844-2011
Preview True-PDF (Reload/Scroll-down if blank)
YY 0844-2011: Laser therapeutic equipment. Pulsed carbon dioxide laser treating instrument
YY 0844-2011
Laser therapeutic equipment.Pulsed carbon dioxide laser treating instrument
ICS 11.040.60
C41
People's Republic of China pharmaceutical industry standards
Laser therapy equipment
Pulsed carbon dioxide laser treatment machine
Issued on. 2011-12-31
2013-06-01 implementation
State Food and Drug Administration issued
Foreword
This standard was drafted in accordance with GB/T 1.1-2009 given rules.
This standard GB 9706.1-2007 "Medical Electrical Equipment Part 1. General requirements for safety", GB 9706.20-2000 "Medical
Electrical equipment - Part 2. diagnostic and therapeutic laser equipment requirements for safety ", GB 7247.1-2001" Safety of Laser Products
Part 1. Equipment Classification, Requirements and User's Guide. "
Please note that some of the content of this document may involve patents. Release mechanism of the present document does not assume responsibility for the identification of these patents.
This standard was proposed by the State Food and Drug Administration.
This standard by the national medical and optical instruments Standardization Technical Committee (SAC/TC103/SC1) centralized.
This standard by the State Food and Drug Administration Hangzhou Medical Device Quality Supervision and Inspection Center, Zhejiang Medical Device Testing, Chongqing
Beijing and Chongqing Laser Technology Co., Ltd. is responsible for drafting.
The main drafters of this standard. Han Jian City, Zhou Zhikang, Du Kun, Ye Yueshun hole Peng.
Laser therapy equipment
Pulsed carbon dioxide laser treatment machine
1 Scope
This standard specifies the pulsed carbon dioxide laser treatment of the basic parameters and the composition of products, technical requirements, test methods, and signs and markings
Sign, packaging, etc., the present standard-setting product standards for the registration of medical devices pulsed carbon dioxide laser treatment machine manufacturer's specifications.
This standard applies to pulsed carbon dioxide laser treatment and containing both continuous wave operation mode contains only pulse operation mode, pulse
Run the carbon dioxide laser treatment machine pulse operation section (hereinafter referred to as the treatment machine). The standard refers to "Pulse", the pulse
Chong duration (pulse width) is less than 0.25s. Treatment machine via a wavelength of 10.6μm pulsed laser vaporization of tissue, carbon
Of freezing and irradiation for therapeutic purposes.
Containing both continuous wave operation mode, pulse operation mode carbon dioxide laser treatment, which should be consistent with the continuous wave operation section
GB 11748, its pulse operation section shall comply with this standard.
2 Normative references
The following documents for the application of this document is essential, dated references, only the dated version suitable for use herein
Member. For undated references, the latest edition (including any amendments) applies to this document.
GB/T 191 Packaging - Pictorial signs
GB 7247.1 Safety of laser products - Part 1. Equipment Classification, Requirements and User's Guide
GB 9706.1 Medical Electrical Equipment Part 1. General requirements for safety
GB 9706.20 Medical electrical equipment - Part 2. diagnostic and therapeutic laser equipment requirements for the safety
GB/T 14710 medical electrical environmental requirements and test methods
GB/T 17736 laser safety goggles main parameters of the test method
YY 91057 medical foot switch General technical conditions
ISO 11146 Test methods for laser and laser-related equipment laser beam widths, divergence angles and beam propagation ratios (Lasersand
Laser-relatedequipmenttestmethodsforlaserbeamwidths, divergenceanglesandbeampropagation
ratios)
3 Terms and Definitions
Terms and definitions defined in GB 7247.1 and the following terms and definitions apply to this document.
3.1
50% of the pulse duration (pulse width of 50%) 50% -pulseduration
τ50
Laser pulse rise time and fall to 50% of its peak power point interval between, see Figure 1.
Figure 1 laser pulse duration (pulse width) and pulse repetition cycle schematic
3.2
10% of the pulse duration (pulse width of 10%) 10% -pulseduration
τ10
Laser pulse rise time and fall to 10% of its peak power point interval between, see Figure 1.
3.3
50% 50% -pulsepower pulse power
P50
P50 is a pulse energy Q pulse duration and 50% (50% pulse width) ratio of τ50, see formula (1).
P50 = Qτ50
(1)
3.4
10% 10% -pulsepower pulse power
P10
Q energy pulse P10 with a pulse duration of 10% (10% pulse width) τ10 ratio, see formula (2).
P10 = Qτ10
(2)
4 product composition and basic parameters
4.1 part of the treatment machine
a) carbon dioxide laser;
b) power and control systems;
c) Security;
d) aim, the transmission system;
e) cooling system;
f) output system and accessories (may include an output handpiece, the scanning device/dot output devices, etc.).
4.2 Treatment of basic parameters
a) therapeutic laser wavelength and mode;
b) treatment laser pulse energy Q output terminal, a burst of energy Q series, the average power Pav, pulse power P50 and P10;
c) treatment of the laser pulse duration (pulse width) τ (τ50 and τ10), the pulse repetition period T or the pulse repetition frequency f or accounting
Air ratio of [eta] (see Fig. 1, Fig. 2);
d) for the duration of the laser pulse train (burst width) T1, the burst interval T2 (see FIG. 2) or burst repetition period
T string; string within the number of pulses n, train within the pulse duration (pulse width) τ (τ50 and τ10), the string within the pulse repetition period T (see
Figures 1, 2);
e) Treatment of laser divergence angle of the output terminal, coke (light) spot diameter;
f) aiming light wavelength;
g) aiming light power.
4.3 Safety Category
Manufacturers should be listed in the following categories registered product safety standards.
a) class and type in accordance with the provisions of GB 9706.1;
b) Type of laser radiation in accordance with the provisions of GB 7247.1.
2 laser pulse output and time parameters Schematic diagram
5 Requirements
5.1 Normal operating conditions
Manufacturers should provide at least the following parameters normal working conditions.
--- Ambient temperature;
---Relative humidity;
---using electric.
5.2 Laser Treatment
5.2.1 laser wavelength
10.6μm ± 0.1μm.
5.2.2 laser mode
Fundamental transverse mode or multi-mode.
Its output laser pulse temporal characteristics 5.2.3 (Figure 1, Figure 2)
5.2.3.1 The manufacturer shall specify the laser pulse output may include (see Figure 2).
a) one-shot pulse output mode;
b) pulse repetition output;
c) a single pulse train output;
d) burst repetition output.
5.2.3.2 laser pulse duration (pulse width) τ50 and τ10, pulse repetition period T or the pulse repetition frequency f or the duty ratio η.
a) If the τ50 by the operator can not adjust, the manufacturer should specify the corresponding output τ50 nominal value and tolerances, or scope of τ50;
And gives the corresponding τ10 nominal value and tolerance, or the range τ10. If T is not available by the operator to adjust, the manufacturer shall specify
The corresponding output nominal value and tolerance, or from T T's; above tolerance shall not exceed ± 20%.
Note. tolerances. refers to the measurement of the actual output minus the value of its nominal value, or set its tolerance nominal value or setpoint (deviation of the actual output of the measurement
Algebraic difference value). Following the same.
b) If τ50 adjusted by the operator, the manufacturer should specify the corresponding output setting range and tolerance τ50 at the same time to give the corresponding
The τ10 scope and tolerance; if T or f or η adjustment by the operator, the manufacturer should specify the corresponding output T or f
Or η setting range and tolerance; above tolerance shall not exceed ± 20%.
5.2.3.3 Laser burst duration (burst width) within the string and the Tl pulse number n (see Figure 2).
a) If the T1, n can not be adjusted by the operator, the manufacturer shall specify the nominal T1 nominal value and tolerance (not to exceed ± 20%) and n
Value, or T1, n ranges;
b) If the T1, n adjustment by the operator, the manufacturer should provide T1 set range and tolerance (not to exceed ± 20%) and setting n
range.
5.2.3.4 Laser burst interval T2 (see FIG. 2) or the burst repetition period T string.
a) If the T2, T string can not be adjusted by the operator, the manufacturer should specify the corresponding output T2 or T series of the nominal value and tolerances, or
T2 or scope of T string; above tolerance shall not exceed ± 20%.
b) If T2 or T series adjusted by the operator, the manufacturer should specify the corresponding output T2 or T string setting range and tolerance;
Above tolerance shall not exceed ± 20%.
5.2.4 laser pulse (train) terminal output energy and power
5.2.4.1 pulse energy Q, Q energy burst string.
a) The manufacturer shall specify the corresponding output pulse energy ratings QR, and gives the corresponding τ50 and τ10; manufacturers should be regulated
Given under the corresponding output pulse train energy rating Q series R, and gives the corresponding n; maximum output of the machine should not treat small
In QR and Q series R;
Note. RATINGS nominal amount of the output of a standard middle finger under the specified conditions of use and performance of the normal operation of the maximum output value. Following the same.
b) If the operator Q can be adjusted, the manufacturer shall specify the scope and Q set tolerance, and gives the corresponding τ50 and τ10; if Q series
Adjusted by the operator, the manufacturer shall specify the Q train set range and tolerance, and gives the corresponding n. Above shall not tolerance
More than ± 20%.
5.2.4.2 Terminal output average power Pav, pulse power P50 and the corresponding P10 (Figure 1, Figure 2).
a) The manufacturer shall specify the average power rating PavR, pulse power rating P50R respective output mode, but given the P50R
Corresponding P10R; maximum output of the machine should not be less than the treatment PavR, P50R;
b) If Pav adjusted by the operator, the manufacturer should specify the corresponding output Pav setting range and allow the difference; if P50 can be operated
On regulation, the manufacturer should specify the corresponding output P50 setting range and allow its poor, and gives the corresponding P10; more
Tolerance should not exceed ± 20%.
5.2.5 terminal laser output power instability St
If the treatment machine has a pulse repetition output and (or) repeatedly outputs a burst mode, the manufacturer should provide power instability St, St
Should not exceed ± 10%.
5.2.6 Terminal laser output power/energy reproducibility RP
If the treatment machine has a pulse repetition output and (or) repeatedly outputs a burst mode, the manufacturer shall specify the level of the corresponding output mode
Average power reproducibility RP, RP should not exceed ± 10%;
If the treatment machine has a one-shot pulse output mode and (or) a single pulse train output, manufacturers should provide appropriate energy output under
Reproducibility RP, RP should not exceed ± 10%.
5.2.7 terminal laser divergence angle and coke (light) spot diameter
The manufacturer shall specify the terminal and the divergence angle of the laser output coke (light) spot diameter and the nominal value of tolerance, tolerance should not exceed ± 20%.
5.3 Targeting System
5.3.1 aiming light wavelength
The manufacturer shall specify the nominal value of the wavelength of light to aim and tolerance (tolerance shall not exceed ± 5nm), or wavelength range.
5.3.2 aiming light output terminal
It should be less than 5mW.
5.4 light guide system
5.4.1 The treatment uses a carbon dioxide laser tube length L> when 500mm, shall be equipped with a light guide arm or other light guide system.
5.4.2 light guide system within the scope of their freedom should be no snags and dead phenomenon.
5.5 cooling system using a cooling liquid
5.5.1 liquid-cooled cooling system should be no leakage.
5.5.2 When using liquid cooling liquid cooling system stop, should be able to automatically cut off the laser power supply.
5.6 laser radiation category
The manufacturer shall in accordance with the provisions of GB 7247.1 laser radiation therapy machine category.
5.7 Output Accessories
Treatment machine has an output accessory, the manufacturer shall specify the characteristics of the various parameters of the laser output accessory, including these attachments work output operation area
Range (or nominal) shape parameters and tolerance, tolerance should not exceed ± 20%.
5.8 Appearance
5.8.1 treatment machine should look neat, uniform color, no corrosion, coating peeling, scars, scratches, deformation and other defects.
5.8.2 treatment machine text and markings should be clearly visible.
5.8.3 Treatment control adjustment mechanism should be flexible and reliable, no loosening the fastening parts, buttons, switches feel clear and reliable operation.
5.9 Laser protective glasses
Therapy machine should be configured to meet the following requirements of laser protection glasses.
5.9.1 protective glasses for laser therapy wavelengths of light density. ≥4.
5.9.2 Visible transmittance. ≥65%.
It shall be marked with the wavelength range and optical density protection 5.9.3 on laser protection glasses.
5.10 footswitch
Shall comply with the requirements of 2.9 and 2.5,2.6,2.7,2.8 YY 91057-1999.
5.11 Safety
Therapy machine should be consistent with GB 9706.1, GB 9706.20, GB 7247.1 requirements of.
The manufacturer shall, respectively, in accordance with GB 9706.1, GB 9706.20, GB 7247.1 provisions of its security features (including an insulating panel) and apply
Provisions.
5.12 environmental adaptability
Environmental adaptability treatment machine should be consistent with GB/T 14710 requirements; manufacturers should give a specific test conditions and test items. test
Project should contain at least 5.2.4.2a) laser average output power rating PavR.
6 Test methods
6.1 Test methods listed in this chapter t...
Share