1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY 1271-2016 English PDF
YY 1271-2016 English PDF
Regular price
$135.00 USD
Regular price
Sale price
$135.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY 1271-2016 (Self-service in 1-minute)
Historical versions (Master-website): YY 1271-2016
Preview True-PDF (Reload/Scroll-down if blank)
YY 1271-2016: Cardiopulmonary bypass systems. Suction catheter for single use
YY 1271-2016
Cardiopulmonary bypass systems.Suction catheter for single use
ICS 11.040.40
C45
People's Republic of China Pharmaceutical Industry Standard
Cardiopulmonary bypass system
Disposable suction tube
Published on.2016-03-23
2018-01-01 Implementation
The State Food and Drug Administration issued
Foreword
All technical content of this standard is mandatory.
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
Please note that some of the contents of this document may involve patents. The issuing agency of this document does not assume responsibility for identifying these patents.
This standard is proposed by the State Food and Drug Administration.
This standard is under the jurisdiction of the National Medical Cardiopulmonary Bypass Equipment Standardization Technical Committee (SAC/TC158).
This standard was drafted by. Dongguan Kewei Medical Instrument Co., Ltd., State Food and Drug Administration Guangzhou Medical Device Quality Supervision
Inspection center.
The main drafters of this standard are Liu Shengsheng, Tang Yunhua, Hong Liangtong, and He Xiaofan.
Cardiopulmonary bypass system
Disposable suction tube
1 Scope
This standard stipulates the classification and structure, requirements, test methods, signs, and signs of aseptic disposable suction tubes (hereinafter referred to as suction tubes).
Signs, instructions for use, packaging, transportation, storage.
This standard applies to suction tubes for supporting cardiopulmonary bypass systems, suction tubes for cardiovascular ventilation in the left heart, suction decompression or
Reduce the load of the left heart and use it to attract liquids such as blood from the heart.
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article
Pieces. For undated references, the latest version (including all amendments) applies to this document.
GB/T 191-2008 packaging, storage and transportation logo
GB/T 9969-2008 Instruction Manual for Industrial Products General
GB/T 14233.1-2008 Medical infusion, blood transfusion, injection equipment inspection methods Part 1. Chemical analysis methods
GB/T 14233.2-2005 Methods for the examination of medical infusions, blood transfusions and syringes - Part 2. Biological methods
GB/T 16886.1 Biological evaluation of medical devices Part 1. Assessment and testing in risk management process
GB 18279 Medical Device Ethylene Oxide Sterilization Verification and Routine Control
GB 18280 Sterilization Confirmation and Routine Control of Healthcare Products Require Radiation Sterilization
GB 19335-2003 General technical conditions for one-time use of blood products
YY/T 0149-2006 Test Method for Corrosion Resistance of Stainless Steel Medical Devices
YY/T 0466.1-2009 Medical Devices Symbols for Labeling, Marking, and Providing Information on Medical Devices Part 1. General Purpose
Claim
YY/T 0681.1-2009 Test Methods for Packaging Sterile Medical Devices Part 1. Accelerated Weathering Test Guide
3 Classification and Structure
3.1 Classification
This standard stipulates that there are two types of left heart drainage tube and heart chamber suction tube (right heart suction tube) according to the use of the product; the left heart drainage tube is mainly used for body
In external circulation heart surgery, it is used for left heart exhaust, decompression or reduction of left heart load, and cardiac cavity suction tube is mainly used in cardiopulmonary bypass heart surgery.
It is used to attract blood and other fluids in the cardiac field and transmit it back to the reservoir.
Suction tube should be product outer diameter or outer circumference length as a specification. Recommended in millimeters (mm) if not in millimeters (mm)
The operating instructions should have a comparison table. The specific specifications, models and dimensions are regulated by the manufacturer according to their actual conditions.
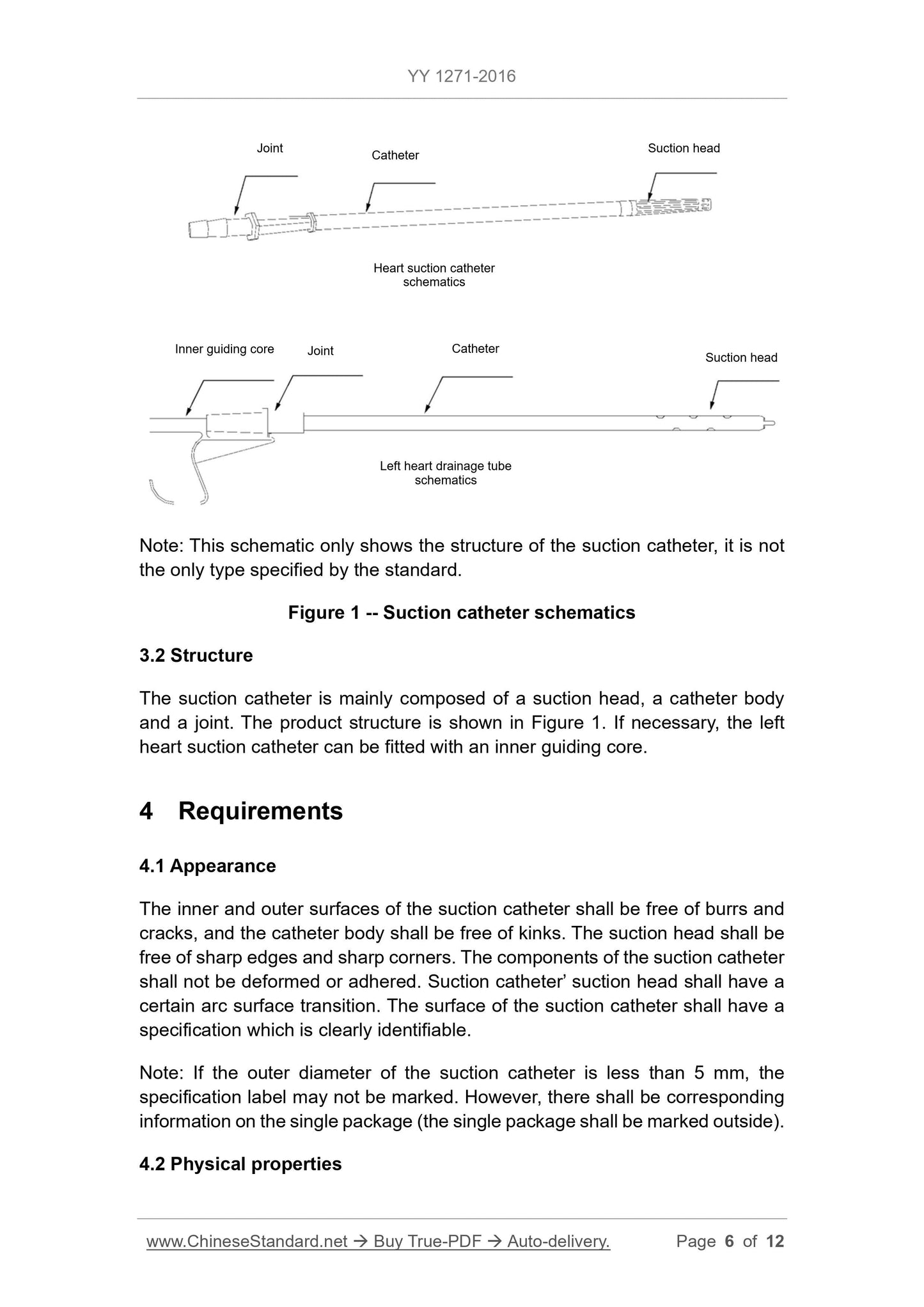
Note. This schematic only shows the structure of the suction tube, not the only type specified for the standard.
Figure 1 Aspiration tube
3.2 Structure
The suction pipe is mainly composed of a suction head, a pipe body and a joint. The product structure is shown in Figure 1. If necessary, the left heart suction tube can be fitted with
Lead core.
4 Requirements
4.1 Appearance
The inner and outer surfaces of the suction tube should be free of burrs and cracks, and the tube body should be free of kinks. The suction head should be free of sharp edges and sharp corners. Suction tube components
The pieces should not be deformed or adhered. Suction tube suction head should have a certain arc surface transition. The surface of the suction tube should have a specification and be clearly identifiable.
Note. If the outer diameter of the suction pipe is less than 5mm, the specification mark may not be marked, but corresponding information shall be provided on the single package (the outside of the single package shall be marked).
4.2 Physical properties
4.2.1 No leakage
Absorption tube should be no leakage phenomenon.
4.2.2 Connection strength
Suction pipe connections should be firmly connected.
4.2.3 Temperature Adaptability
The suction tube should be free from deformation and rupture in the temperature range of 0°C to 50°C.
4.2.4 Negative pressure tolerance
Under room temperature conditions, a negative pressure of 20 kPa (150 mmHg) is applied for 15 seconds, and the suction tube body should not be flattened.
4.3 Biological properties
4.3.1 Biological Evaluation
Suction tubes should be free of biological hazards.
4.3.2 Sterility
The suction tube should be sterilized by the sterilization process that has been confirmed.
4.3.3 Non-pyrogenic
The suction tube should be pyrogen free.
4.4 Chemical properties
4.4.1 Reducing substances (easily oxides)
The difference between the volume of the 20mL test solution and the potassium permanganate solution [c(KMnO4)=0.002mol/L] consumed in the same batch of blank control solution
Should not exceed 2.0 mL.
4.4.2 Heavy Metals
When measured by atomic absorption spectrophotometry (AAS) or equivalent method, the total content of antimony, chromium, copper, lead, and tin in the test solution
Should not exceed 1 μg/mL. The content of cadmium should not exceed 0.1 μg/mL.
When tested by colorimetry, the test solution should exhibit a color that does not exceed the standard control solution with a mass concentration of ρ(Pb2)=1 μg/mL.
4.4.3 pH
Check the liquid and the same batch of blank liquid, the difference between the pH should not exceed 1.5.
4.4.4 Evaporation Residue
The total amount of evaporation residue of the 50 mL test solution should not exceed 2 mg.
4.4.5 UV absorbance
The absorbance of the test solution should not exceed 0.1.
4.4.6 Color
The test solution should be colorless and transparent.
4.4.7 Ethylene Oxide Residue
When the suction tube is sterilized with ethylene oxide gas, the residual ethylene oxide content should not exceed 10 mg/kg.
4.5 Corrosion resistance
If there are metal parts, the corrosion resistance of the metal parts shall not exceed class b.
4.6 Particle Pollution
The number of particles 15μm~25μm on the surface area per square centimeter of a suction tube must not exceed 1, and the number of particles larger than 25μm does not
More than 0.5.
4.7 Validity period
The period of validity shall be given, and the product shall meet the specified requirements during the period of validity.
5 test methods
5.1 Appearance
With visual observation, the suction tube should meet the requirements of 4.1.
5.2 Physical Performance
5.2.1 No leakage
Connect the aspiration tube passage, pass air or nitrogen gas above atmospheric pressure by 50 kPa, place it in water for 10 min, observe carefully.
Bubbles escape. Meet the requirements of 4.2.1.
5.2.2 Connection strength
Axial static tensile force of 15 N is applied at each joint of the suction tube for 15 s. Separation should not occur and the requirements of 4.2.2 should be met.
5.2.3 Temperature Adaptability
The suction tube was placed in a 0°C environment for 3 minutes, then placed in a 50°C environment for 3 hours, and then taken out and returned to room temperature for observation.
The non-leakage test of 5.2.1 shall comply with the provisions of 4.2.3.
5.2.4 Negative pressure tolerance
The suction end of the suction tube is connected to a vacuum source to block the suction hole, and a negative pressure of 20 kPa is applied for 15 seconds at room temperature to suck the tube of the tube.
The body should not occur flat ticks, should comply with the provisions of 4.2.4.
5.3 Biological properties
5.3.1 Biological Evaluation
The biological properties should be evaluated according to the provisions of GB/T 16886.1.
5.3.2 Sterility test
The sterilization process should be confirmed according to relevant standards such as GB 18279 or GB 18280.
Sterility test according to the provisions of GB/T 14233.2-2005, the method should not be used for factory inspection.
5.3.3 Pyrogen Test
According to the provisions of GB/T 14233.2-2005 for inspection, shall comply with the provisions of 4.3.3.
5.4 Chemical properties
5.4.1 Preparation of test solution
According to the provisions of GB/T 14233.1-2008 in Table 1 No. 1 preparation test solution.
5.4.2 Reducing Substance (Easy Oxide) Test
According to the provisions of the method in 5.2.2 of GB/T 14233.1-2008, shall comply with the provisions of 4.4.1.
5.4.3 Heavy Metals
Tests in accordance with the provisions of 5.6.1 and 5.9.1 of GB/T 14233.1-2008 shall comply with the requirements of 4.4.2.
5.4.4 pH test
According to the provisions of 5.4.1 of GB/T 14233.1-2008, it shall comply with the provisions of 4.4.3.
5.4.5 Evaporation Residue Test
According to the provisions of 5.5 in GB/T 14233.1-2008, it shall comply with the provisions of 4.4.4.
5.4.6 UV Absorbance Test
According to GB/T 14233.1-2008 in the wavelength range of 250nm ~ 320nm, should comply with the provisions of 4.4.5.
5.4.7 Color
Visual inspection liquids should meet the requirements of 4.4.6.
5.4.8 Ethylene Oxide Residue
According to the provisions of GB/T 14233.1-2008 for inspection, shall comply with the provisions of 4.4.7
5.5 Corrosion resistance
Remove the metal parts, according to YY/T 0149-2006 sodium chloride solution test method, the results should comply with the provisions of 4.5.
5.6 Particle Pollution
Test according to the provisions of Appendix A of GB 19335-2003 for testing, shall comply with the provisions of 4.6.
5.7 Validity test
Take products that have expired not more than one month (Arbitration Act, should be preferred), or perform aging according to YY/T 0681.1-2009.
Test 4.2, 4.3.2, 4.3.3, the results should meet the requirements of 4.7.
6 Signs, Instruction Manual
6.1 Logo
6.1.1 The product packaging should have the following symbols.
a) the name of the manufacturer;
b) product name, model specifications;
c) production lot number, validity period;
d) "One-time use", "sterility", "breakage of packaging prohibited," "please read the manual before use," or other words or illustrations.
6.1.2 Product packaging should have the following signs.
a) Manufacturer's name, address;
b) product name, model specifications;
c) implementation of the standard number;
d) product registration number;
e) production license number;
f) production lot number;
g) "One-time use" or other words or illustrations;
h) Sterilization methods;
i) Validity period;
j) Package quantity, volume (length × width × height);
k) "Handle with care", "Do not press" or "Fat to fear" words or signs, shall comply with GB/T 191-2008, YY/T 0466.1-2009
In the relevant regulations, the words or signs should be guaranteed not to be blurred due to longer duration.
6.2 Instruction Manual
6.2.1 One instruction manual should be attached to the suction tube package.
6.2.2 The preparation of the operating instructions shall comply with the relevant provisions of GB/T 9969-2008.
7 Packaging, Transport, Storage
7.1 Packaging
Each suction tube should have a single package.
A number of small packages are large packages that are packed into a single box.
7.2 Transport
Manufacturers shall make their own regulations according to actual conditions.
7.3 Storage
The suction tube after packaging should be stored at a temperature of 0°C to 40°C, a relative humidity of no more than 80%, no corrosion gas, and good ventilation
indoor.
Get Quotation: Click YY 1271-2016 (Self-service in 1-minute)
Historical versions (Master-website): YY 1271-2016
Preview True-PDF (Reload/Scroll-down if blank)
YY 1271-2016: Cardiopulmonary bypass systems. Suction catheter for single use
YY 1271-2016
Cardiopulmonary bypass systems.Suction catheter for single use
ICS 11.040.40
C45
People's Republic of China Pharmaceutical Industry Standard
Cardiopulmonary bypass system
Disposable suction tube
Published on.2016-03-23
2018-01-01 Implementation
The State Food and Drug Administration issued
Foreword
All technical content of this standard is mandatory.
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
Please note that some of the contents of this document may involve patents. The issuing agency of this document does not assume responsibility for identifying these patents.
This standard is proposed by the State Food and Drug Administration.
This standard is under the jurisdiction of the National Medical Cardiopulmonary Bypass Equipment Standardization Technical Committee (SAC/TC158).
This standard was drafted by. Dongguan Kewei Medical Instrument Co., Ltd., State Food and Drug Administration Guangzhou Medical Device Quality Supervision
Inspection center.
The main drafters of this standard are Liu Shengsheng, Tang Yunhua, Hong Liangtong, and He Xiaofan.
Cardiopulmonary bypass system
Disposable suction tube
1 Scope
This standard stipulates the classification and structure, requirements, test methods, signs, and signs of aseptic disposable suction tubes (hereinafter referred to as suction tubes).
Signs, instructions for use, packaging, transportation, storage.
This standard applies to suction tubes for supporting cardiopulmonary bypass systems, suction tubes for cardiovascular ventilation in the left heart, suction decompression or
Reduce the load of the left heart and use it to attract liquids such as blood from the heart.
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article
Pieces. For undated references, the latest version (including all amendments) applies to this document.
GB/T 191-2008 packaging, storage and transportation logo
GB/T 9969-2008 Instruction Manual for Industrial Products General
GB/T 14233.1-2008 Medical infusion, blood transfusion, injection equipment inspection methods Part 1. Chemical analysis methods
GB/T 14233.2-2005 Methods for the examination of medical infusions, blood transfusions and syringes - Part 2. Biological methods
GB/T 16886.1 Biological evaluation of medical devices Part 1. Assessment and testing in risk management process
GB 18279 Medical Device Ethylene Oxide Sterilization Verification and Routine Control
GB 18280 Sterilization Confirmation and Routine Control of Healthcare Products Require Radiation Sterilization
GB 19335-2003 General technical conditions for one-time use of blood products
YY/T 0149-2006 Test Method for Corrosion Resistance of Stainless Steel Medical Devices
YY/T 0466.1-2009 Medical Devices Symbols for Labeling, Marking, and Providing Information on Medical Devices Part 1. General Purpose
Claim
YY/T 0681.1-2009 Test Methods for Packaging Sterile Medical Devices Part 1. Accelerated Weathering Test Guide
3 Classification and Structure
3.1 Classification
This standard stipulates that there are two types of left heart drainage tube and heart chamber suction tube (right heart suction tube) according to the use of the product; the left heart drainage tube is mainly used for body
In external circulation heart surgery, it is used for left heart exhaust, decompression or reduction of left heart load, and cardiac cavity suction tube is mainly used in cardiopulmonary bypass heart surgery.
It is used to attract blood and other fluids in the cardiac field and transmit it back to the reservoir.
Suction tube should be product outer diameter or outer circumference length as a specification. Recommended in millimeters (mm) if not in millimeters (mm)
The operating instructions should have a comparison table. The specific specifications, models and dimensions are regulated by the manufacturer according to their actual conditions.
Note. This schematic only shows the structure of the suction tube, not the only type specified for the standard.
Figure 1 Aspiration tube
3.2 Structure
The suction pipe is mainly composed of a suction head, a pipe body and a joint. The product structure is shown in Figure 1. If necessary, the left heart suction tube can be fitted with
Lead core.
4 Requirements
4.1 Appearance
The inner and outer surfaces of the suction tube should be free of burrs and cracks, and the tube body should be free of kinks. The suction head should be free of sharp edges and sharp corners. Suction tube components
The pieces should not be deformed or adhered. Suction tube suction head should have a certain arc surface transition. The surface of the suction tube should have a specification and be clearly identifiable.
Note. If the outer diameter of the suction pipe is less than 5mm, the specification mark may not be marked, but corresponding information shall be provided on the single package (the outside of the single package shall be marked).
4.2 Physical properties
4.2.1 No leakage
Absorption tube should be no leakage phenomenon.
4.2.2 Connection strength
Suction pipe connections should be firmly connected.
4.2.3 Temperature Adaptability
The suction tube should be free from deformation and rupture in the temperature range of 0°C to 50°C.
4.2.4 Negative pressure tolerance
Under room temperature conditions, a negative pressure of 20 kPa (150 mmHg) is applied for 15 seconds, and the suction tube body should not be flattened.
4.3 Biological properties
4.3.1 Biological Evaluation
Suction tubes should be free of biological hazards.
4.3.2 Sterility
The suction tube should be sterilized by the sterilization process that has been confirmed.
4.3.3 Non-pyrogenic
The suction tube should be pyrogen free.
4.4 Chemical properties
4.4.1 Reducing substances (easily oxides)
The difference between the volume of the 20mL test solution and the potassium permanganate solution [c(KMnO4)=0.002mol/L] consumed in the same batch of blank control solution
Should not exceed 2.0 mL.
4.4.2 Heavy Metals
When measured by atomic absorption spectrophotometry (AAS) or equivalent method, the total content of antimony, chromium, copper, lead, and tin in the test solution
Should not exceed 1 μg/mL. The content of cadmium should not exceed 0.1 μg/mL.
When tested by colorimetry, the test solution should exhibit a color that does not exceed the standard control solution with a mass concentration of ρ(Pb2)=1 μg/mL.
4.4.3 pH
Check the liquid and the same batch of blank liquid, the difference between the pH should not exceed 1.5.
4.4.4 Evaporation Residue
The total amount of evaporation residue of the 50 mL test solution should not exceed 2 mg.
4.4.5 UV absorbance
The absorbance of the test solution should not exceed 0.1.
4.4.6 Color
The test solution should be colorless and transparent.
4.4.7 Ethylene Oxide Residue
When the suction tube is sterilized with ethylene oxide gas, the residual ethylene oxide content should not exceed 10 mg/kg.
4.5 Corrosion resistance
If there are metal parts, the corrosion resistance of the metal parts shall not exceed class b.
4.6 Particle Pollution
The number of particles 15μm~25μm on the surface area per square centimeter of a suction tube must not exceed 1, and the number of particles larger than 25μm does not
More than 0.5.
4.7 Validity period
The period of validity shall be given, and the product shall meet the specified requirements during the period of validity.
5 test methods
5.1 Appearance
With visual observation, the suction tube should meet the requirements of 4.1.
5.2 Physical Performance
5.2.1 No leakage
Connect the aspiration tube passage, pass air or nitrogen gas above atmospheric pressure by 50 kPa, place it in water for 10 min, observe carefully.
Bubbles escape. Meet the requirements of 4.2.1.
5.2.2 Connection strength
Axial static tensile force of 15 N is applied at each joint of the suction tube for 15 s. Separation should not occur and the requirements of 4.2.2 should be met.
5.2.3 Temperature Adaptability
The suction tube was placed in a 0°C environment for 3 minutes, then placed in a 50°C environment for 3 hours, and then taken out and returned to room temperature for observation.
The non-leakage test of 5.2.1 shall comply with the provisions of 4.2.3.
5.2.4 Negative pressure tolerance
The suction end of the suction tube is connected to a vacuum source to block the suction hole, and a negative pressure of 20 kPa is applied for 15 seconds at room temperature to suck the tube of the tube.
The body should not occur flat ticks, should comply with the provisions of 4.2.4.
5.3 Biological properties
5.3.1 Biological Evaluation
The biological properties should be evaluated according to the provisions of GB/T 16886.1.
5.3.2 Sterility test
The sterilization process should be confirmed according to relevant standards such as GB 18279 or GB 18280.
Sterility test according to the provisions of GB/T 14233.2-2005, the method should not be used for factory inspection.
5.3.3 Pyrogen Test
According to the provisions of GB/T 14233.2-2005 for inspection, shall comply with the provisions of 4.3.3.
5.4 Chemical properties
5.4.1 Preparation of test solution
According to the provisions of GB/T 14233.1-2008 in Table 1 No. 1 preparation test solution.
5.4.2 Reducing Substance (Easy Oxide) Test
According to the provisions of the method in 5.2.2 of GB/T 14233.1-2008, shall comply with the provisions of 4.4.1.
5.4.3 Heavy Metals
Tests in accordance with the provisions of 5.6.1 and 5.9.1 of GB/T 14233.1-2008 shall comply with the requirements of 4.4.2.
5.4.4 pH test
According to the provisions of 5.4.1 of GB/T 14233.1-2008, it shall comply with the provisions of 4.4.3.
5.4.5 Evaporation Residue Test
According to the provisions of 5.5 in GB/T 14233.1-2008, it shall comply with the provisions of 4.4.4.
5.4.6 UV Absorbance Test
According to GB/T 14233.1-2008 in the wavelength range of 250nm ~ 320nm, should comply with the provisions of 4.4.5.
5.4.7 Color
Visual inspection liquids should meet the requirements of 4.4.6.
5.4.8 Ethylene Oxide Residue
According to the provisions of GB/T 14233.1-2008 for inspection, shall comply with the provisions of 4.4.7
5.5 Corrosion resistance
Remove the metal parts, according to YY/T 0149-2006 sodium chloride solution test method, the results should comply with the provisions of 4.5.
5.6 Particle Pollution
Test according to the provisions of Appendix A of GB 19335-2003 for testing, shall comply with the provisions of 4.6.
5.7 Validity test
Take products that have expired not more than one month (Arbitration Act, should be preferred), or perform aging according to YY/T 0681.1-2009.
Test 4.2, 4.3.2, 4.3.3, the results should meet the requirements of 4.7.
6 Signs, Instruction Manual
6.1 Logo
6.1.1 The product packaging should have the following symbols.
a) the name of the manufacturer;
b) product name, model specifications;
c) production lot number, validity period;
d) "One-time use", "sterility", "breakage of packaging prohibited," "please read the manual before use," or other words or illustrations.
6.1.2 Product packaging should have the following signs.
a) Manufacturer's name, address;
b) product name, model specifications;
c) implementation of the standard number;
d) product registration number;
e) production license number;
f) production lot number;
g) "One-time use" or other words or illustrations;
h) Sterilization methods;
i) Validity period;
j) Package quantity, volume (length × width × height);
k) "Handle with care", "Do not press" or "Fat to fear" words or signs, shall comply with GB/T 191-2008, YY/T 0466.1-2009
In the relevant regulations, the words or signs should be guaranteed not to be blurred due to longer duration.
6.2 Instruction Manual
6.2.1 One instruction manual should be attached to the suction tube package.
6.2.2 The preparation of the operating instructions shall comply with the relevant provisions of GB/T 9969-2008.
7 Packaging, Transport, Storage
7.1 Packaging
Each suction tube should have a single package.
A number of small packages are large packages that are packed into a single box.
7.2 Transport
Manufacturers shall make their own regulations according to actual conditions.
7.3 Storage
The suction tube after packaging should be stored at a temperature of 0°C to 40°C, a relative humidity of no more than 80%, no corrosion gas, and good ventilation
indoor.
Share