1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 0079-2016 English PDF (YYT0079-2016)
YY/T 0079-2016 English PDF (YYT0079-2016)
Regular price
$130.00 USD
Regular price
Sale price
$130.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY/T 0079-2016 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 0079-2016
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 0079-2016: Metallic clip
YY/T 0079-2016
Metallic clip
ICS 11.040.30
C36
People's Republic of China Pharmaceutical Industry Standard
Replacing YY/T 0079-2006
Medical metal clip
Metalicclip
Published on.2016-03-23
2017-01-01 Implementation
The State Food and Drug Administration issued
Foreword
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
This standard replaces YY/T 0079-2006 "Surgical Implant Metal Clips."
The main differences between this standard and YY/T 0079-2006 are as follows.
--- Changed the name of the standard, changed from "Surgical implant metal clip" to "medical metal clip";
--- Revised structural style and size requirements;
--- Revised the material grade and hardness range of titanium metal clips;
--- Delete enamel metal clips and related content;
--- Increased clamping performance, corrosion resistance and package sealing requirements and test methods;
--- Increase the requirements of microbiological indicators and test methods;
--- Improve biocompatibility requirements and test methods.
Please note that some of the contents of this document may involve patents. The issuing agency of this document does not assume responsibility for identifying these patents.
This standard was proposed and managed by the National Surgical Instruments Standardization Technical Committee (SAC/TC94).
This standard was drafted by. Hangzhou Tonglu Medical Optical Instrument Factory, Shanghai Medical Device Testing Institute, Johnson and Johnson (Shanghai) Medical Equipment Co., Ltd.
Limited company.
Participants in this standard. Xinhua Surgical Instruments Co., Ltd.
Drafters of this standard. Shen Tuyuhua, Zheng Qiang, Shi Wanxia, Huang Shuze, Yu Rong.
The previous versions of the standards replaced by this standard are.
---WSZ-196-1975;
---YY 0079.1-1992;
---YY/T 0079-2006.
Medical metal clip
1 Scope
This standard specifies the type and basic dimensions of metal clips, requirements, test methods, inspection rules, markings, operating instructions, packaging, transportation,
Storage requirements.
This standard applies to silver clips that clamp the capillaries of the brain and titanium clips that clamp the abdominal tubular tissue. The following are referred to as (metal clips).
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article
Pieces. For undated references, the latest version (including all amendments) applies to this document.
GB/T 4135 Silver Bar
GB/T 4340.1 Vickers hardness test of metallic materials Part 1. Test methods
GB/T 6682 Analysis Laboratory Water Specifications and Test Methods
GB/T 13810-2007 titanium and titanium alloys for surgical implants
GB/T 14233.2-2005 Methods for the examination of medical infusions, blood transfusions and syringes - Part 2. Biological methods
GB/T 16886.3-2008 Biological evaluation of medical devices - Part 3. Genetic toxicity, carcinogenicity and reproductive toxicity tests
GB/T 16886.5-2003 Biological evaluation of medical devices Part 5. In vitro cytotoxicity test
GB/T 16886.6-1997 Biological evaluation of medical devices - Part 6. Local response test after implantation
GB/T 16886.7 Biological evaluation of medical devices. Part 7. Ethylene oxide sterilization residue
GB/T 16886.10-2005 Biological evaluation of medical devices - Part 10. Stimulation and delayed type hypersensitivity tests
GB/T 16886.11-2011 Biological evaluation of medical devices Part 11. Systemic toxicity test
YY/T 0149-2006 Test Method for Corrosion Resistance of Stainless Steel Medical Devices
YY/T 0171-2008 Surgical device packaging, signs and instructions
YY/T 0466.1 Medical Devices Symbols for Labeling, Marking, and Providing Information on Medical Devices Part 1. General Requirements
YY/T 0597-2006 Application clamp
YY/T 0681.4 Test methods for packaging sterile medical devices. Part 4. Dyeing fluid penetration method for leak detection of leaks in breathable packages
Pharmacopoeia of the People's Republic of China (2010 Edition, Part 2)
3 type and basic size
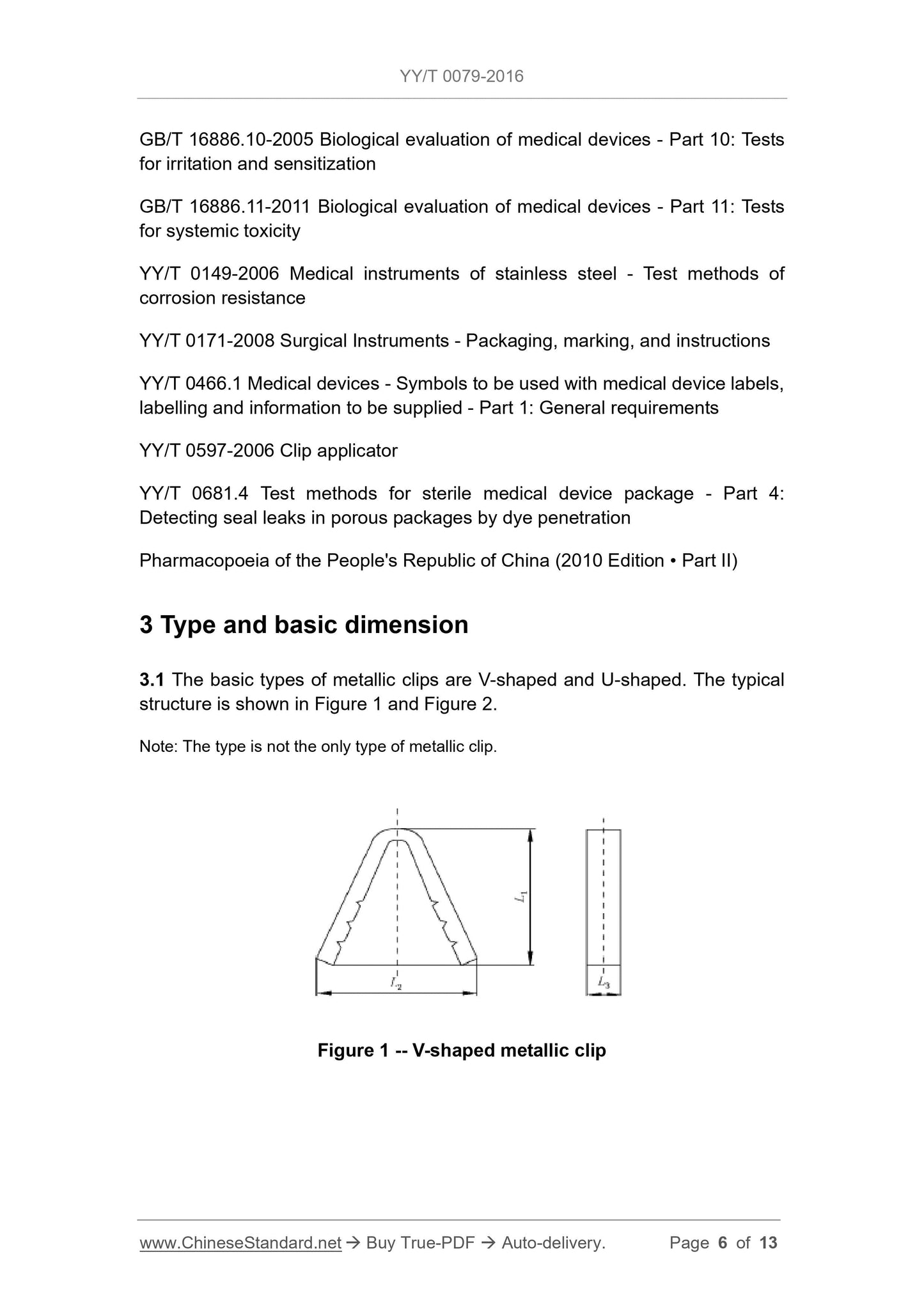
3.1 The basic types of metal clips are V-shaped and U-shaped. Their typical structures are shown in Figure 1 and Figure 2.
Note. The only type of this type of non-metallic clip.
Figure 1 V-shaped metal clips
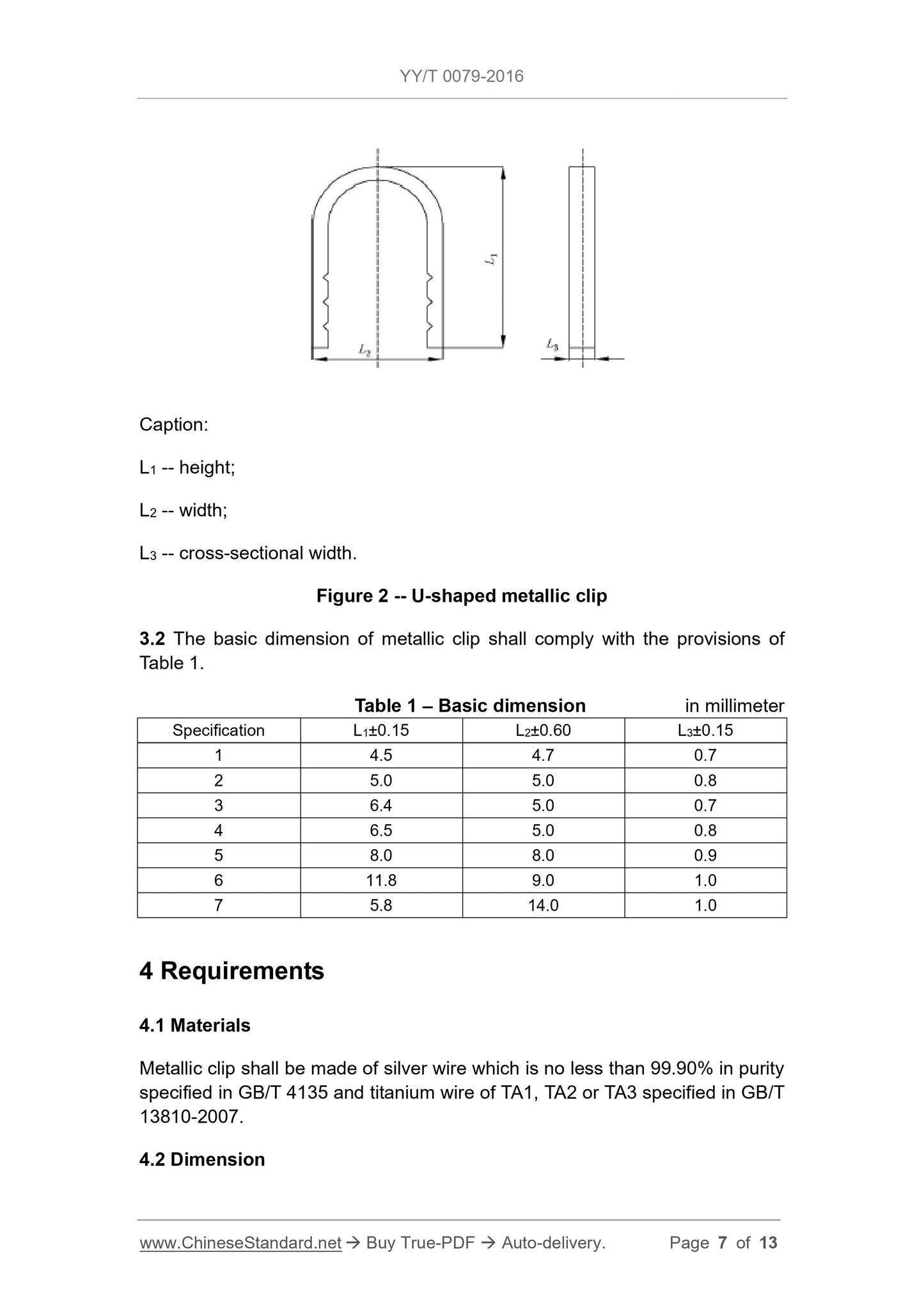
Explanation.
L1 --- height;
L2---width;
L3 - cross-sectional width.
Figure 2 U-shaped metal clips schematic
3.2 The basic dimensions of the metal clips are to comply with the requirements of Table 1.
Table 1 The basic dimensions are in millimeters
Specifications L1±0.15 L2±0.60 L3±0.15
1 4.5 4.7 0.7
2 5.0 5.0 0.8
3 6.4 5.0 0.7
4 6.5 5.0 0.8
5 8.0 8.0 0.9
6 11.8 9.0 1.0
7 5.8 14.0 1.0
4 Requirements
4.1 Materials
The metal clip shall have a purity of not less than 99.90% of the silver wire specified in GB/T 4135 and a TA1 as specified in GB/T 13810-2007.
TA2 or TA3 titanium wire material.
4.2 Size
The dimensions of the metal clips L1, L2, L3 shall comply with the provisions of 3.2.
4.3 Appearance
4.3.1 Both sides of the metal clip should be symmetrical, and the outer shape should be flat, with no defects such as sharp edges, burrs, cracks, and spots.
4.3.2 The stripes and/or grooves on the metal clip should be clear and complete, and there should be no overflow or sharp edges.
4.4 Surface roughness
The surface roughness parameter Ra of the metal clip should not exceed 0.8 μm.
4.5 Hardness
The hardness of the silver metal clip is 60HV0.1 to 100HV0.1, and the hardness of the titanium metal clip is 100HV0.2 to.200HV0.2.
4.6 Toughness
Metal clips should have good toughness and must not be cracked or broken after being clamped.
4.7 Clipping performance
The metal clamp and clamp clamp are well-matched and should have good clamping performance.
4.8 Corrosion resistance
The metal clip should have good corrosion resistance, and no trace of corrosion will be produced after the test.
4.9 Sterility
If the metal clip is delivered in a sterile state, it should be subjected to an effective, validated sterilization process to make the product sterile.
4.10 Microbiological indicators
If the metal clip is delivered in a non-sterile state, its initial contamination should be no more than 100 CFU/piece.
4.11 Ethylene oxide residue
If the metal clip is sterilized with ethylene oxide gas, the residual amount should not exceed 10 μg/g.
4.12 Packaging Seal
Aseptically packaged metal clips should be sealed in a single package and have good resistance to bacteria.
4.13 Biocompatibility
4.13.1 The cytotoxicity of metal clips should not be greater than grade 1.
4.13.2 The metal clamp sensitization reaction should not be greater than 1.
4.13.3 The internal reaction of the metal clip should not be greater than 1.
4.13.4 Metal clips should have no acute systemic toxicity.
4.13.5 Metal clips should have no subchronic toxicity.
4.13.6 Metal clips should have no genetic toxicity.
4.13.7 Metal clips should have no implant reaction.
4.13.8 Bacterial endotoxin in aseptically packaged silver clips should not exceed 2.15 EU/piece.
5 test methods
5.1 Material Inspection
Chemical composition analysis method of silver metal clips is carried out according to the method specified in GB/T 4135, chemical composition analysis method for titanium metal clips
According to the method specified in GB/T 13810-2007, it shall comply with the provisions of 4.1.
5.2 Inspection of dimensions
Testing with universal gauges should meet the requirements of 4.2.
5.3 appearance inspection
Under conditions of 5x to 10x magnification, normal or corrected visual acuity should meet the requirements of 4.3.1 and 4.3.2.
5.4 Surface roughness test
The surface roughness of the metal clip is tested by the sample comparison method and compared with the sample under magnification of 5 to 10 times, using normal or corrected
Visual inspection should comply with the provisions of 4.4.
5.5 Hardness
According to the provisions of GB/T 4340.1, measuring 3 points on the metal clip, calculate the arithmetic mean, should comply with the provisions of 4.5.
5.6 Toughness test
After clamping the metal clamp with a clamp clamp that matches the metal clamp and conforms to YY/T 0597-2006, use a 5x to 10x magnifying glass.
Observations should meet the requirements of 4.6.
5.7 Clamping performance test
According to the provisions in Table 2 of YY/T 0597-2006, select test materials, match with metal clips, in line with YY/T 0597-2006
The clamps are clamped and the clamps are clamped onto the suspended test material. Loosen the clamp jaws. The metal clamp should be able to be fixed on the test material. No relative
Displacement shall meet the requirements of 4.7.
5.8 Corrosion resistance test
Silver clips were tested according to YY/T 0149-2006 sodium chloride solution test method.
Titanium clip, using sodium hydroxide 0.005g, plus three test water 250mL in accordance with GB/T 6682, the solution temperature is 20 °C ±
5°C, stir well. It was immersed in the solution and kept for 48 h and dried.
Use a 5x to 10x magnifier to check that the test results meet the requirements of 4.8.
5.9 Sterility test
According to the Pharmacopoeia of the People's Republic of China (2010 Edition, Part II) in the inspection of Appendix XI H sterility test, should meet the 4.9
Claim.
5.10 Microbial indicators
According to the "Chinese Pharmacopoeia" (2010 Edition, Part II) in the Appendix XIJ microbial limit test method for inspection, shall comply with 4.10
Provisions.
5.11 Ethylene Oxide Residue
Detection according to the method specified in GB/T 16886.7, shall comply with the provisions of 4.11.
Preparation of the test solution. The metal clips and the test water were configured in a ratio of 1.0 g.5 mL, and sealed and equilibrated at a temperature of 60° C.±1° C. for 40 minutes.
5.12 Packaging Seal Inspection
According to the method specified in YY/T 0681.4, it shall comply with the provisions of 4.12.
5.13 Biocompatibility
5.13.1 Cytotoxicity Test
According to GB/T 16886.5-2003 8.2 (extract liquid test) prescribed method, should meet the requirements of 4.13.1.
5.13.2 Sensitization, intradermal reaction test
According to the methods specified in 7.4 (maximum dose test) and Appendix B.2 (intradermal reaction test) in GB/T 16886.10-2005,
Meet the requirements of 4.13.2 and 4.13.3.
5.13.3 Assessment of Systemic and Subchronic Toxicity
According to GB/T 16886.11-2011 in Chapter 5 and Chapter 6 methods for evaluation, should meet the requirements of 4.13.4 and 4.13.5.
5.13.4 Genetic toxicity assessment
According to GB/T 16886.3-2008 4.4 provisions of the method of evaluation, should meet the requirements of 4.13.6.
5.13.5 Evaluation of Implantation Response
Evaluation according to the method specified in 3.4 of GB/T 16886.6-1997 shall meet the requirements of 4.13.7.
5.13.6 Bacterial endotoxin
According to the method specified in GB/T 14233.2-2005, it shall comply with the provisions of 4.13.8.
6 Inspection Rules
Type inspection items are those specified in Chapter 4, 7.1.1, and 7.1.2.
If there is no special provision, the items to be inspected shall be randomly sampled by 3 and all shall be qualified.
7 Signs, Instruction Manual
7.1 Logo
7.1.1 Ordinary single package shall have at least the material code specified in Table 3 and the mark specified in 4.1.1 of YY/T 0171-2008.
7.1.2 Sterile single package shall have at least the material code specified in Table 3 and the mark specified in 4.1.3 of YY/T 0171-2008.
7.1.3 The outer package shall have at least the mark specified in 4.2.2 of YY/T 0171-2008. The material code is shown in Table 2.
Table 2 Material Codes
Material code
Silver Ag
Titanium Ti
7.1.4 The certificate shall comply with the relevant provisions of YY/T 0466.1.
7.2 Instruction Manual
The instructions for use shall comply with 5.2, 5.3 and 5.4 of YY/T 0171-2008.
8 Packaging and Storage
8.1 Packaging
8.1.1 Metal clips are generally packaged in accordance with 3.1 of YY/T 0171-2008.
8.1.2 Aseptic packaging of metal clips shall comply with 3.3 of YY/T 0171-2008 and the following provisions.
a) The metal clips are aseptically packaged and should remain sterile during the storage period.
b) The metal clips are aseptically packaged and should be left open after opening.
8.1.3 Single packaging into the box should be accompanied by instruction manual and certificate of conformity.
8.2 Transport, Storage and Validity
8.2.1 The outer packaging of the metal clip should be able to guarantee no damage under normal conditions of transportation and storage.
8.2.2 The words or signs on the outer packaging should ensure that it should not be long and ambiguous.
8.2.3 The metal clip after packaging should be stored in a room with a relative humidity of no more than 80%, no corrosive gas and good ventilation. Metal clips from sterilization
The date of validity shall be not less than two years.
Get Quotation: Click YY/T 0079-2016 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 0079-2016
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 0079-2016: Metallic clip
YY/T 0079-2016
Metallic clip
ICS 11.040.30
C36
People's Republic of China Pharmaceutical Industry Standard
Replacing YY/T 0079-2006
Medical metal clip
Metalicclip
Published on.2016-03-23
2017-01-01 Implementation
The State Food and Drug Administration issued
Foreword
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
This standard replaces YY/T 0079-2006 "Surgical Implant Metal Clips."
The main differences between this standard and YY/T 0079-2006 are as follows.
--- Changed the name of the standard, changed from "Surgical implant metal clip" to "medical metal clip";
--- Revised structural style and size requirements;
--- Revised the material grade and hardness range of titanium metal clips;
--- Delete enamel metal clips and related content;
--- Increased clamping performance, corrosion resistance and package sealing requirements and test methods;
--- Increase the requirements of microbiological indicators and test methods;
--- Improve biocompatibility requirements and test methods.
Please note that some of the contents of this document may involve patents. The issuing agency of this document does not assume responsibility for identifying these patents.
This standard was proposed and managed by the National Surgical Instruments Standardization Technical Committee (SAC/TC94).
This standard was drafted by. Hangzhou Tonglu Medical Optical Instrument Factory, Shanghai Medical Device Testing Institute, Johnson and Johnson (Shanghai) Medical Equipment Co., Ltd.
Limited company.
Participants in this standard. Xinhua Surgical Instruments Co., Ltd.
Drafters of this standard. Shen Tuyuhua, Zheng Qiang, Shi Wanxia, Huang Shuze, Yu Rong.
The previous versions of the standards replaced by this standard are.
---WSZ-196-1975;
---YY 0079.1-1992;
---YY/T 0079-2006.
Medical metal clip
1 Scope
This standard specifies the type and basic dimensions of metal clips, requirements, test methods, inspection rules, markings, operating instructions, packaging, transportation,
Storage requirements.
This standard applies to silver clips that clamp the capillaries of the brain and titanium clips that clamp the abdominal tubular tissue. The following are referred to as (metal clips).
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article
Pieces. For undated references, the latest version (including all amendments) applies to this document.
GB/T 4135 Silver Bar
GB/T 4340.1 Vickers hardness test of metallic materials Part 1. Test methods
GB/T 6682 Analysis Laboratory Water Specifications and Test Methods
GB/T 13810-2007 titanium and titanium alloys for surgical implants
GB/T 14233.2-2005 Methods for the examination of medical infusions, blood transfusions and syringes - Part 2. Biological methods
GB/T 16886.3-2008 Biological evaluation of medical devices - Part 3. Genetic toxicity, carcinogenicity and reproductive toxicity tests
GB/T 16886.5-2003 Biological evaluation of medical devices Part 5. In vitro cytotoxicity test
GB/T 16886.6-1997 Biological evaluation of medical devices - Part 6. Local response test after implantation
GB/T 16886.7 Biological evaluation of medical devices. Part 7. Ethylene oxide sterilization residue
GB/T 16886.10-2005 Biological evaluation of medical devices - Part 10. Stimulation and delayed type hypersensitivity tests
GB/T 16886.11-2011 Biological evaluation of medical devices Part 11. Systemic toxicity test
YY/T 0149-2006 Test Method for Corrosion Resistance of Stainless Steel Medical Devices
YY/T 0171-2008 Surgical device packaging, signs and instructions
YY/T 0466.1 Medical Devices Symbols for Labeling, Marking, and Providing Information on Medical Devices Part 1. General Requirements
YY/T 0597-2006 Application clamp
YY/T 0681.4 Test methods for packaging sterile medical devices. Part 4. Dyeing fluid penetration method for leak detection of leaks in breathable packages
Pharmacopoeia of the People's Republic of China (2010 Edition, Part 2)
3 type and basic size
3.1 The basic types of metal clips are V-shaped and U-shaped. Their typical structures are shown in Figure 1 and Figure 2.
Note. The only type of this type of non-metallic clip.
Figure 1 V-shaped metal clips
Explanation.
L1 --- height;
L2---width;
L3 - cross-sectional width.
Figure 2 U-shaped metal clips schematic
3.2 The basic dimensions of the metal clips are to comply with the requirements of Table 1.
Table 1 The basic dimensions are in millimeters
Specifications L1±0.15 L2±0.60 L3±0.15
1 4.5 4.7 0.7
2 5.0 5.0 0.8
3 6.4 5.0 0.7
4 6.5 5.0 0.8
5 8.0 8.0 0.9
6 11.8 9.0 1.0
7 5.8 14.0 1.0
4 Requirements
4.1 Materials
The metal clip shall have a purity of not less than 99.90% of the silver wire specified in GB/T 4135 and a TA1 as specified in GB/T 13810-2007.
TA2 or TA3 titanium wire material.
4.2 Size
The dimensions of the metal clips L1, L2, L3 shall comply with the provisions of 3.2.
4.3 Appearance
4.3.1 Both sides of the metal clip should be symmetrical, and the outer shape should be flat, with no defects such as sharp edges, burrs, cracks, and spots.
4.3.2 The stripes and/or grooves on the metal clip should be clear and complete, and there should be no overflow or sharp edges.
4.4 Surface roughness
The surface roughness parameter Ra of the metal clip should not exceed 0.8 μm.
4.5 Hardness
The hardness of the silver metal clip is 60HV0.1 to 100HV0.1, and the hardness of the titanium metal clip is 100HV0.2 to.200HV0.2.
4.6 Toughness
Metal clips should have good toughness and must not be cracked or broken after being clamped.
4.7 Clipping performance
The metal clamp and clamp clamp are well-matched and should have good clamping performance.
4.8 Corrosion resistance
The metal clip should have good corrosion resistance, and no trace of corrosion will be produced after the test.
4.9 Sterility
If the metal clip is delivered in a sterile state, it should be subjected to an effective, validated sterilization process to make the product sterile.
4.10 Microbiological indicators
If the metal clip is delivered in a non-sterile state, its initial contamination should be no more than 100 CFU/piece.
4.11 Ethylene oxide residue
If the metal clip is sterilized with ethylene oxide gas, the residual amount should not exceed 10 μg/g.
4.12 Packaging Seal
Aseptically packaged metal clips should be sealed in a single package and have good resistance to bacteria.
4.13 Biocompatibility
4.13.1 The cytotoxicity of metal clips should not be greater than grade 1.
4.13.2 The metal clamp sensitization reaction should not be greater than 1.
4.13.3 The internal reaction of the metal clip should not be greater than 1.
4.13.4 Metal clips should have no acute systemic toxicity.
4.13.5 Metal clips should have no subchronic toxicity.
4.13.6 Metal clips should have no genetic toxicity.
4.13.7 Metal clips should have no implant reaction.
4.13.8 Bacterial endotoxin in aseptically packaged silver clips should not exceed 2.15 EU/piece.
5 test methods
5.1 Material Inspection
Chemical composition analysis method of silver metal clips is carried out according to the method specified in GB/T 4135, chemical composition analysis method for titanium metal clips
According to the method specified in GB/T 13810-2007, it shall comply with the provisions of 4.1.
5.2 Inspection of dimensions
Testing with universal gauges should meet the requirements of 4.2.
5.3 appearance inspection
Under conditions of 5x to 10x magnification, normal or corrected visual acuity should meet the requirements of 4.3.1 and 4.3.2.
5.4 Surface roughness test
The surface roughness of the metal clip is tested by the sample comparison method and compared with the sample under magnification of 5 to 10 times, using normal or corrected
Visual inspection should comply with the provisions of 4.4.
5.5 Hardness
According to the provisions of GB/T 4340.1, measuring 3 points on the metal clip, calculate the arithmetic mean, should comply with the provisions of 4.5.
5.6 Toughness test
After clamping the metal clamp with a clamp clamp that matches the metal clamp and conforms to YY/T 0597-2006, use a 5x to 10x magnifying glass.
Observations should meet the requirements of 4.6.
5.7 Clamping performance test
According to the provisions in Table 2 of YY/T 0597-2006, select test materials, match with metal clips, in line with YY/T 0597-2006
The clamps are clamped and the clamps are clamped onto the suspended test material. Loosen the clamp jaws. The metal clamp should be able to be fixed on the test material. No relative
Displacement shall meet the requirements of 4.7.
5.8 Corrosion resistance test
Silver clips were tested according to YY/T 0149-2006 sodium chloride solution test method.
Titanium clip, using sodium hydroxide 0.005g, plus three test water 250mL in accordance with GB/T 6682, the solution temperature is 20 °C ±
5°C, stir well. It was immersed in the solution and kept for 48 h and dried.
Use a 5x to 10x magnifier to check that the test results meet the requirements of 4.8.
5.9 Sterility test
According to the Pharmacopoeia of the People's Republic of China (2010 Edition, Part II) in the inspection of Appendix XI H sterility test, should meet the 4.9
Claim.
5.10 Microbial indicators
According to the "Chinese Pharmacopoeia" (2010 Edition, Part II) in the Appendix XIJ microbial limit test method for inspection, shall comply with 4.10
Provisions.
5.11 Ethylene Oxide Residue
Detection according to the method specified in GB/T 16886.7, shall comply with the provisions of 4.11.
Preparation of the test solution. The metal clips and the test water were configured in a ratio of 1.0 g.5 mL, and sealed and equilibrated at a temperature of 60° C.±1° C. for 40 minutes.
5.12 Packaging Seal Inspection
According to the method specified in YY/T 0681.4, it shall comply with the provisions of 4.12.
5.13 Biocompatibility
5.13.1 Cytotoxicity Test
According to GB/T 16886.5-2003 8.2 (extract liquid test) prescribed method, should meet the requirements of 4.13.1.
5.13.2 Sensitization, intradermal reaction test
According to the methods specified in 7.4 (maximum dose test) and Appendix B.2 (intradermal reaction test) in GB/T 16886.10-2005,
Meet the requirements of 4.13.2 and 4.13.3.
5.13.3 Assessment of Systemic and Subchronic Toxicity
According to GB/T 16886.11-2011 in Chapter 5 and Chapter 6 methods for evaluation, should meet the requirements of 4.13.4 and 4.13.5.
5.13.4 Genetic toxicity assessment
According to GB/T 16886.3-2008 4.4 provisions of the method of evaluation, should meet the requirements of 4.13.6.
5.13.5 Evaluation of Implantation Response
Evaluation according to the method specified in 3.4 of GB/T 16886.6-1997 shall meet the requirements of 4.13.7.
5.13.6 Bacterial endotoxin
According to the method specified in GB/T 14233.2-2005, it shall comply with the provisions of 4.13.8.
6 Inspection Rules
Type inspection items are those specified in Chapter 4, 7.1.1, and 7.1.2.
If there is no special provision, the items to be inspected shall be randomly sampled by 3 and all shall be qualified.
7 Signs, Instruction Manual
7.1 Logo
7.1.1 Ordinary single package shall have at least the material code specified in Table 3 and the mark specified in 4.1.1 of YY/T 0171-2008.
7.1.2 Sterile single package shall have at least the material code specified in Table 3 and the mark specified in 4.1.3 of YY/T 0171-2008.
7.1.3 The outer package shall have at least the mark specified in 4.2.2 of YY/T 0171-2008. The material code is shown in Table 2.
Table 2 Material Codes
Material code
Silver Ag
Titanium Ti
7.1.4 The certificate shall comply with the relevant provisions of YY/T 0466.1.
7.2 Instruction Manual
The instructions for use shall comply with 5.2, 5.3 and 5.4 of YY/T 0171-2008.
8 Packaging and Storage
8.1 Packaging
8.1.1 Metal clips are generally packaged in accordance with 3.1 of YY/T 0171-2008.
8.1.2 Aseptic packaging of metal clips shall comply with 3.3 of YY/T 0171-2008 and the following provisions.
a) The metal clips are aseptically packaged and should remain sterile during the storage period.
b) The metal clips are aseptically packaged and should be left open after opening.
8.1.3 Single packaging into the box should be accompanied by instruction manual and certificate of conformity.
8.2 Transport, Storage and Validity
8.2.1 The outer packaging of the metal clip should be able to guarantee no damage under normal conditions of transportation and storage.
8.2.2 The words or signs on the outer packaging should ensure that it should not be long and ambiguous.
8.2.3 The metal clip after packaging should be stored in a room with a relative humidity of no more than 80%, no corrosive gas and good ventilation. Metal clips from sterilization
The date of validity shall be not less than two years.
Share