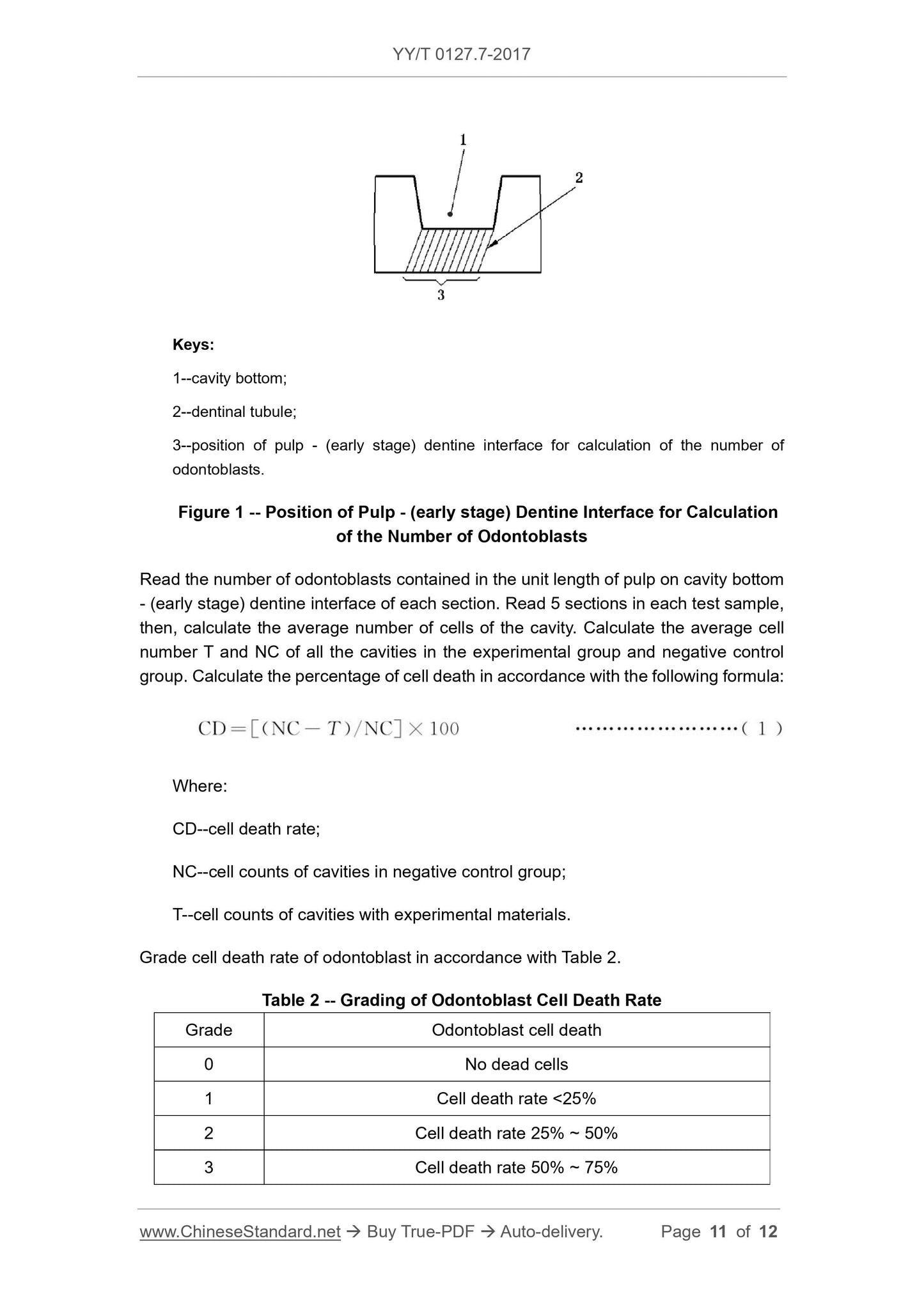
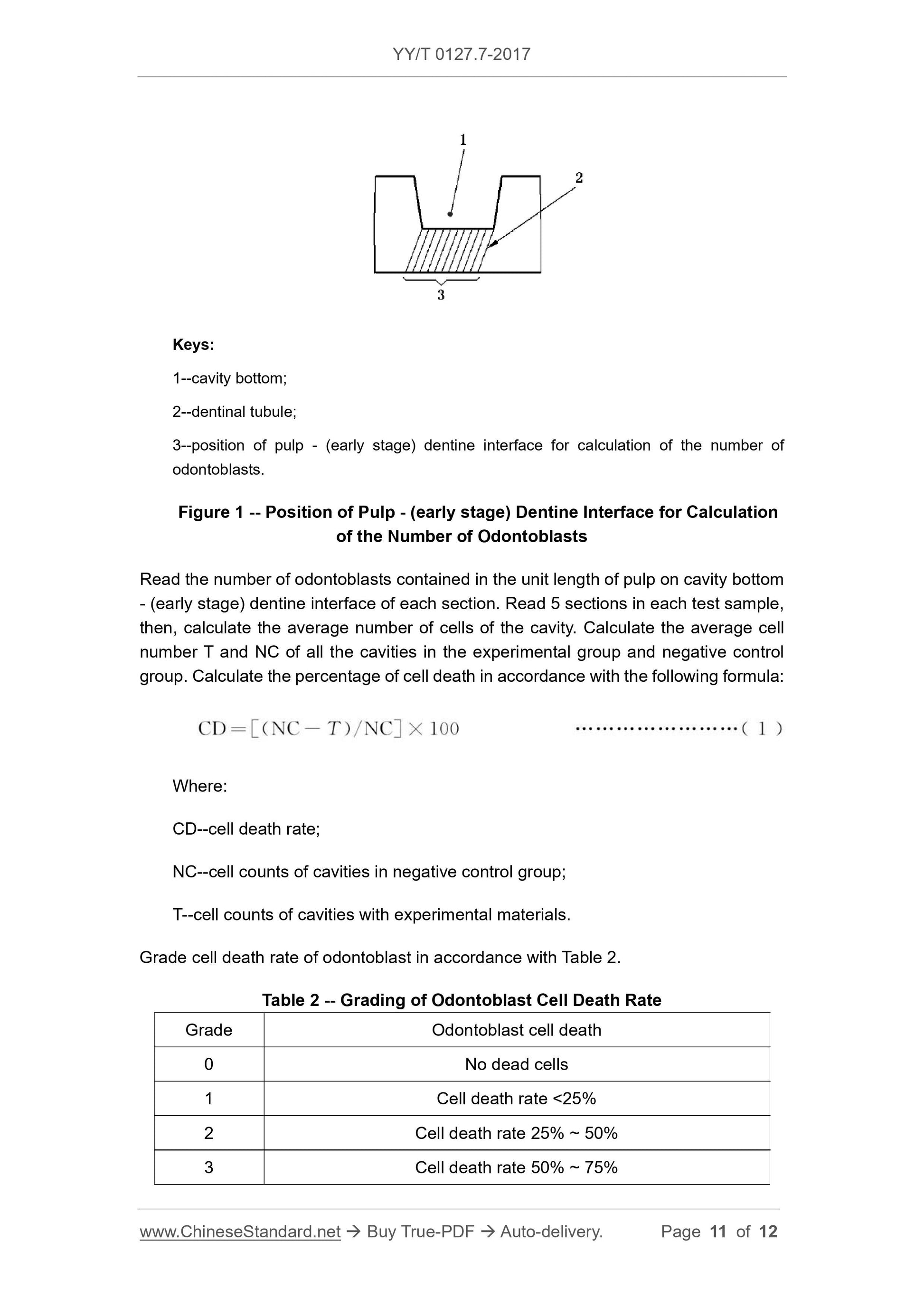
1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 0127.7-2017 English PDF (YYT0127.7-2017)
YY/T 0127.7-2017 English PDF (YYT0127.7-2017)
Regular price
$130.00 USD
Regular price
Sale price
$130.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY/T 0127.7-2017 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 0127.7-2017
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 0127.7-2017: Biological evaluation of dental materials -- Part 2: Biological evaluation test method of dental materials -- Pulp and dentine usage test
YY/T 0127.7-2017
Biological evaluation of dental materials -- Part 2. Biological evaluation test method of dental materials -- Pulp and dentine usage test
ICS 11.060.10
C33
People's Republic of China Pharmaceutical Industry Standard
Replacing YY/T 0127.7-2001
Biological evaluation of oral medical devices
Part 7. Endodontic application test
Part 7. Pulpanddentineusagetest
(ISO 7405.2008, Dentistry-Evaluationofbiocompatibilityofmedical
2017-03-28 released.2018-04-01 implementation
State Food and Drug Administration issued
Foreword
This part is drafted in accordance with the rules given in GB/T 1.1-2009.
The YY/T 0127 series of standards are the standards for specific biological test methods for oral medical devices, which are divided into the following sections.
---YY/T 0127.1 Oral material biological test method hemolysis test
---YY/T 0127.2 Biological evaluation of oral medical devices Unit 2. Test methods Acute systemic toxicity test. vein
way
---YY/T 0127.3 Biological evaluation of oral medical devices - Part 3
---YY/T 0127.4 Biological evaluation of oral medical devices Part 2. Biological test methods for oral materials Bone implant test
---YY/T 0127.5 Biological evaluation of oral medical devices - Part 5. Inhalation toxicity test
---YY/T 0127.6 Biological evaluation of oral materials - Module 2. Biological test method for oral materials
---YY/T 0127.7 Biological evaluation of oral medical devices - Part 7. Tests for the application of dental pulp dentin
---YY/T 0127.8 Biological evaluation of oral materials Part 2. Biological test methods for oral materials Subcutaneous implantation test
---YY/T 0127.9 Biological Evaluation of Oral Materials Unit 2. Biological Test Methods for Oral Materials Cytotoxicity Test. Qiong
Lipid coating method and molecular filtration method
---YY/T 0127.10 Biological evaluation of oral medical devices Unit 2. Test method Salmonella typhimurium recovery
Variable test (Ames test)
---YY/T 0127.11 Biological evaluation of oral medical devices - Part 11. Capsule test
---YY/T 0127.12 Dentistry oral medical device biological evaluation unit 2. test method micronucleus test
---YY/T 0127.13 Biological evaluation of oral medical devices Unit 2. Test methods Oral mucosal irritation test
---YY/T 0127.14 Biological evaluation of oral medical devices Part 2. Test methods Acute oral systemic toxicity test
---YY/T 0127.15 Biological evaluation of oral medical devices Unit 2. Test methods Subacute and subchronic systemic toxicity
Test. oral route
---YY/T 0127.16 Biological evaluation of oral medical devices Unit 2. Test methods In vitro chromosomes of mammalian cells
Distortion test
---YY/T 0127.17 Biological evaluation of oral medical devices - Part 17. Mutation of mouse lymphoma cells (TK)
This part is the seventh part of the YY/T 0127 series of standards.
This part replaces YY/T 0127.7-2001 "Body Material Biology Evaluation Part 2. Dental Materials Biological Test Method Teeth
Endodontic application test.
The main technical changes in this section and YY/T 0127.7-2001 are as follows, and these changes are based on ISO 7405.2008.
modify.
--- Cancel the era in the normative reference file, delete the "YY 0272-1995 dental zinc oxide butyl" in the normative reference file
"Phenol phenol cement" standard.
--- "Negative control material" is described in detail.
--- Added "Note" to the "positive control".
--- Added the content of "animal welfare" in Chapter 6, "Laboratory Animals." The selection of experimental animals has increased ferrets.
--- The thickness of the remaining dentin at the bottom of the tooth cavity is increased by "preferably less than 0.5mm".
--- Added 8.2.2 "Test Preparation" content.
--- Change the thickness of the tissue section from 5μm to 10μm to 5μm~7μm.
--- Change the evaluation index from 0 to 3 to 0 to 4 (Table 1).
--- Added the content of "Odontoblast cell survival analysis".
--- Deleted the contents of 11.1~11.5 in the evaluation of YY 0127.7-2001 results.
This section uses the redrafting method to refer to ISO 7405.2008 "Diagnostics for the biocompatibility evaluation of dental devices for oral use".
The degree of conformity with ISO 7405.2008 is non-equivalent.
Please note that some of the contents of this document may involve patents. The issuing organization of this document is not responsible for identifying these patents.
This standard was proposed by the State Food and Drug Administration.
This standard is under the jurisdiction of the National Technical Committee for Standardization of Dental Materials and Devices (SAC/TC99).
This standard was drafted. Peking University Medical Device Quality Supervision and Inspection Center of the State Food and Drug Administration.
The main drafters of this standard. Lin Hong, Han Jianmin, Yue Lin.
The previous versions of the standards replaced by this standard are.
---YY/T 0127.7-2001.
Biological evaluation of oral medical devices
Part 7. Endodontic application test
1 Scope
This part of YY/T 0127 specifies the test method for the application of dental pulp dentin.
This section is used to evaluate the biocompatibility of dental materials with dentin and pulp, including the evaluations required for the intended clinical application of the material.
Price method steps.
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article.
Pieces. For undated references, the latest edition (including all amendments) applies to this document.
GB/T 16886.2 Biological evaluation of medical devices - Part 2. Animal welfare requirements (GB/T 16886.2-2011,
ISO 10993-2.2006, IDT)
3 negative control materials
The fast-curing zinc oxide eugenol cement is a suitable negative control material. For long-term research, it is best to protect zinc oxide cloves
Phenol cement to prevent its hydrolysis, can be coated on the surface of zinc oxide eugenol cement with a thin layer of zinc carboxylate cement or traditional (self-condensing) glass
The sub-gate is prevented from hydrolyzing and then filled with a resin-based composite material which is etched and fixed. Need to pay attention to the zinc oxide eugenol watergate
Direct contact with the resin-based composite prevents the polymerization of the resin-based composite. Traditional glass ionomer cement can also be considered as a yin
Sexual control material.
4 positive control
Filling materials or techniques used on unexposed pulp can cause moderate to severe reactions in the pulp to be a suitable positive control (eg silicon)
Water cement).
Note. Repair materials or techniques that do not involve pulp exposure and can continue to cause moderate to severe pulpal reactions are suitable positive controls.
5 Animal and animal welfare
5.1 Animal welfare
Animal welfare should be as follows.
a) GB/T 16886.2 or;
b) National regulatory requirements for laboratory animals.
Note. Feeding according to GB/T 16886.2, and free access to food and water.
5.2 Experimental animals
Using the same line of non-rodent mammals, the appropriate age for the animal is that the dentition contains the apex that has formed (the apical mature)
Constant teeth.
Monkeys, dogs, miniature pigs and ferrets are suitable animal lines. Other germplasm animals can be used for special purposes. Animal species should be selected at the most
Meet the minimum requirements for scientific research with low animal welfare costs. Animal selection should be demonstrated and documented.
If monkeys, dogs or miniature pigs are used, use at least 1 animal per test cycle. If you use a ferrets, because only the canines are suitable
At least 3 animals are used for each test cycle.
Note. Suitable monkeys, dogs and miniature pigs mean that all permanent teeth except M3 have erupted. A suitable ferrets means that 4 permanent fangs have erupted because only the fangs are
Be applicable.
6 test cycle
7d ± 2d, 28d ± 3d and 70d ± 5d.
7 Test procedure
7.1 Animal preparation
Choose enough animals to ensure that at least 7 healthy teeth in each test cycle contain test materials and 4 teeth contain negative controls
material. Animals were anesthetized and tested as described in 7.2.
7.2 Dental preparation
7.2.1 Cavity preparation
Remove all tartar and soft dirt from the tooth surface. Clean and disinfect the tooth surface with 3% (volume fraction) hydrogen peroxide, then use iodine or wash
Bite disinfectant disinfection. Prepare the required number of V-type holes on the buccal or labial side of the teeth with a sharp burr with sufficient water vapor spray. Place
The residual dentin thickness at the bottom of the cavity is less than 1 mm, preferably less than 0.5 mm, but does not expose the pulp. Except for filling test materials, different requirements
In addition to the operation, the water is used to clean the cavity and the cavity is dried with absorbent cotton.
Note 1. If the animal has obvious inflammation of the gums, the calculus and soft scale should be removed a few days before the cavity is prepared, and even the cleaning should be repeated until the gum inflammation is obtained.
control.
Note 2. In the process of cavity preparation, a calibrated impedance measuring instrument, such as a apex instrument, can be used to assist in estimating the residual dentin thickness.
7.2.2 Preparation of test materials
Prepare the test materials according to the product instructions. If the product specification requires the use of a liner material or a cavity treatment agent (such as dentin bonding)
(agent), then add these operations according to the manufacturer's instructions.
7.2.3 Cavity filling
According to the principle of random distribution, at least 7 holes were filled with test materials for each test period, and 4 nests were filled with negative control materials.
hole. If necessary (see note), there should be 4 positive control sockets per test cycle.
Note. Laboratories with a previous positive control database do not have to do a positive control test unless a positive response is sometimes required.
7.2.4 Postoperative observation and care
It is recommended to observe the animal status at least once a day after surgery and record abnormal performance. Take appropriate measures to change postoperative dietary habits, inflammation
The pain or pain caused by the disease or infection is minimized. Analgesics are given as needed after surgery.
7.3 Slice preparation
7.3.1 Drawing and fixing
Sufficient animals were sacrificed with excess anesthetics or other widely accepted substances at 7d ± 2d, 28d ± 3d and 70d ± 5d
At least 7 teeth in each test cycle were tested with test materials. Check the filling, teeth and supporting tissues to record any anomalies. Take off
Each tissue block containing the treated tooth and its surrounding soft and hard support tissue is fixed with a suitable fixative.
Note. Before the animals are sacrificed, the tissue infusion is performed with a fixative before the tissue block is removed, and the fixation effect is better.
7.3.2 Slicing and dyeing
After fixation, the teeth are decalcified with a suitable decalcifying agent [eg 10% (volume fraction) formic acid or 0.5 mol/LEDTA solution at pH 7.4].
Serial sections were prepared. The teeth are sliced along the long axis of the tooth through the cavity of the tooth, and the thickness is 5 μm to 7 μm. Hematoxylin-eosin (HE) staining, necessary
The stains were taken at intervals using appropriate bacterial staining methods (such as Brown and Brenn) or other methods for testing bacteria.
7.4 Dentin and pulp evaluation
The slice is blindly examined (not known as a test sample or a control sample). For each serial section, comprehensively describe and record dentin, pulp
And all histological features of the periapical tissue, including any histological changes that may be caused by cavity preparation. From serial slices, through
At least 5 sections of the fossa are equally spaced to analyze the inflammatory condition. According to Table 1, the surface pulp tissue (including the odontoblast layer, no fine
The inflammatory infiltration of the cell area and the rich cell area) and the remaining pulp tissue (deep tissue) were graded.
Table 1 Classification criteria for dental pulp dentin application test
Inflammation graded inflammation change description
0 No inflammation. the structure of the pulp tissue adjacent to the bottom of the cavity through the dentinal tubule is normal.
1 mild inflammation. the pulp tissue adjacent to the bottom of the cavity through the dentin tubules is normal, scattered inflammatory cells
2 Moderate inflammation. The normal structure of the pulp tissue adjacent to the bottom of the dentin is still present, and there is a small inflammatory cell aggregation lesion.
3 Severe inflammation. loss of pulp tissue structure through the dentinal tubule and the bottom of the cavity, extensive infiltration of inflammatory cells
4 Abscess formation or extensive inflammatory cell infiltration into tissue regions other than pulp tissue adjacent to the dentinal tubules
When evaluating each slice, record the minimum thickness of the remaining dentin, including measuring the bottom of the hole from the vertical angle to the endodontic - (pre-) dentin boundary
The distance of the face and the distance measured along the direction of the dentinal tubules. The latter means that when the slice plane and the dentinal tubules travel incompletely
When the time comes, that each dentinal tubule in the area fails to extend from the bottom of the hole to the endodontic- (pre-) dentin interface, it should follow the dentinal tubule
The general direction of the line is measured. Calculate the inflammatory response index for each test period, ie, add the scores of the grades obtained for each slice.
Take the total number of slices observed.
The test data shall be separately listed for the test materials (including the recommended liner material and cavity treatment agent), the negative control and the positive pair.
The result of the photo. For the cavity filled with the positive control material, the data previously used under the same test conditions can be used. In addition, record each test
The periodic test material and the control material...
Get Quotation: Click YY/T 0127.7-2017 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 0127.7-2017
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 0127.7-2017: Biological evaluation of dental materials -- Part 2: Biological evaluation test method of dental materials -- Pulp and dentine usage test
YY/T 0127.7-2017
Biological evaluation of dental materials -- Part 2. Biological evaluation test method of dental materials -- Pulp and dentine usage test
ICS 11.060.10
C33
People's Republic of China Pharmaceutical Industry Standard
Replacing YY/T 0127.7-2001
Biological evaluation of oral medical devices
Part 7. Endodontic application test
Part 7. Pulpanddentineusagetest
(ISO 7405.2008, Dentistry-Evaluationofbiocompatibilityofmedical
2017-03-28 released.2018-04-01 implementation
State Food and Drug Administration issued
Foreword
This part is drafted in accordance with the rules given in GB/T 1.1-2009.
The YY/T 0127 series of standards are the standards for specific biological test methods for oral medical devices, which are divided into the following sections.
---YY/T 0127.1 Oral material biological test method hemolysis test
---YY/T 0127.2 Biological evaluation of oral medical devices Unit 2. Test methods Acute systemic toxicity test. vein
way
---YY/T 0127.3 Biological evaluation of oral medical devices - Part 3
---YY/T 0127.4 Biological evaluation of oral medical devices Part 2. Biological test methods for oral materials Bone implant test
---YY/T 0127.5 Biological evaluation of oral medical devices - Part 5. Inhalation toxicity test
---YY/T 0127.6 Biological evaluation of oral materials - Module 2. Biological test method for oral materials
---YY/T 0127.7 Biological evaluation of oral medical devices - Part 7. Tests for the application of dental pulp dentin
---YY/T 0127.8 Biological evaluation of oral materials Part 2. Biological test methods for oral materials Subcutaneous implantation test
---YY/T 0127.9 Biological Evaluation of Oral Materials Unit 2. Biological Test Methods for Oral Materials Cytotoxicity Test. Qiong
Lipid coating method and molecular filtration method
---YY/T 0127.10 Biological evaluation of oral medical devices Unit 2. Test method Salmonella typhimurium recovery
Variable test (Ames test)
---YY/T 0127.11 Biological evaluation of oral medical devices - Part 11. Capsule test
---YY/T 0127.12 Dentistry oral medical device biological evaluation unit 2. test method micronucleus test
---YY/T 0127.13 Biological evaluation of oral medical devices Unit 2. Test methods Oral mucosal irritation test
---YY/T 0127.14 Biological evaluation of oral medical devices Part 2. Test methods Acute oral systemic toxicity test
---YY/T 0127.15 Biological evaluation of oral medical devices Unit 2. Test methods Subacute and subchronic systemic toxicity
Test. oral route
---YY/T 0127.16 Biological evaluation of oral medical devices Unit 2. Test methods In vitro chromosomes of mammalian cells
Distortion test
---YY/T 0127.17 Biological evaluation of oral medical devices - Part 17. Mutation of mouse lymphoma cells (TK)
This part is the seventh part of the YY/T 0127 series of standards.
This part replaces YY/T 0127.7-2001 "Body Material Biology Evaluation Part 2. Dental Materials Biological Test Method Teeth
Endodontic application test.
The main technical changes in this section and YY/T 0127.7-2001 are as follows, and these changes are based on ISO 7405.2008.
modify.
--- Cancel the era in the normative reference file, delete the "YY 0272-1995 dental zinc oxide butyl" in the normative reference file
"Phenol phenol cement" standard.
--- "Negative control material" is described in detail.
--- Added "Note" to the "positive control".
--- Added the content of "animal welfare" in Chapter 6, "Laboratory Animals." The selection of experimental animals has increased ferrets.
--- The thickness of the remaining dentin at the bottom of the tooth cavity is increased by "preferably less than 0.5mm".
--- Added 8.2.2 "Test Preparation" content.
--- Change the thickness of the tissue section from 5μm to 10μm to 5μm~7μm.
--- Change the evaluation index from 0 to 3 to 0 to 4 (Table 1).
--- Added the content of "Odontoblast cell survival analysis".
--- Deleted the contents of 11.1~11.5 in the evaluation of YY 0127.7-2001 results.
This section uses the redrafting method to refer to ISO 7405.2008 "Diagnostics for the biocompatibility evaluation of dental devices for oral use".
The degree of conformity with ISO 7405.2008 is non-equivalent.
Please note that some of the contents of this document may involve patents. The issuing organization of this document is not responsible for identifying these patents.
This standard was proposed by the State Food and Drug Administration.
This standard is under the jurisdiction of the National Technical Committee for Standardization of Dental Materials and Devices (SAC/TC99).
This standard was drafted. Peking University Medical Device Quality Supervision and Inspection Center of the State Food and Drug Administration.
The main drafters of this standard. Lin Hong, Han Jianmin, Yue Lin.
The previous versions of the standards replaced by this standard are.
---YY/T 0127.7-2001.
Biological evaluation of oral medical devices
Part 7. Endodontic application test
1 Scope
This part of YY/T 0127 specifies the test method for the application of dental pulp dentin.
This section is used to evaluate the biocompatibility of dental materials with dentin and pulp, including the evaluations required for the intended clinical application of the material.
Price method steps.
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article.
Pieces. For undated references, the latest edition (including all amendments) applies to this document.
GB/T 16886.2 Biological evaluation of medical devices - Part 2. Animal welfare requirements (GB/T 16886.2-2011,
ISO 10993-2.2006, IDT)
3 negative control materials
The fast-curing zinc oxide eugenol cement is a suitable negative control material. For long-term research, it is best to protect zinc oxide cloves
Phenol cement to prevent its hydrolysis, can be coated on the surface of zinc oxide eugenol cement with a thin layer of zinc carboxylate cement or traditional (self-condensing) glass
The sub-gate is prevented from hydrolyzing and then filled with a resin-based composite material which is etched and fixed. Need to pay attention to the zinc oxide eugenol watergate
Direct contact with the resin-based composite prevents the polymerization of the resin-based composite. Traditional glass ionomer cement can also be considered as a yin
Sexual control material.
4 positive control
Filling materials or techniques used on unexposed pulp can cause moderate to severe reactions in the pulp to be a suitable positive control (eg silicon)
Water cement).
Note. Repair materials or techniques that do not involve pulp exposure and can continue to cause moderate to severe pulpal reactions are suitable positive controls.
5 Animal and animal welfare
5.1 Animal welfare
Animal welfare should be as follows.
a) GB/T 16886.2 or;
b) National regulatory requirements for laboratory animals.
Note. Feeding according to GB/T 16886.2, and free access to food and water.
5.2 Experimental animals
Using the same line of non-rodent mammals, the appropriate age for the animal is that the dentition contains the apex that has formed (the apical mature)
Constant teeth.
Monkeys, dogs, miniature pigs and ferrets are suitable animal lines. Other germplasm animals can be used for special purposes. Animal species should be selected at the most
Meet the minimum requirements for scientific research with low animal welfare costs. Animal selection should be demonstrated and documented.
If monkeys, dogs or miniature pigs are used, use at least 1 animal per test cycle. If you use a ferrets, because only the canines are suitable
At least 3 animals are used for each test cycle.
Note. Suitable monkeys, dogs and miniature pigs mean that all permanent teeth except M3 have erupted. A suitable ferrets means that 4 permanent fangs have erupted because only the fangs are
Be applicable.
6 test cycle
7d ± 2d, 28d ± 3d and 70d ± 5d.
7 Test procedure
7.1 Animal preparation
Choose enough animals to ensure that at least 7 healthy teeth in each test cycle contain test materials and 4 teeth contain negative controls
material. Animals were anesthetized and tested as described in 7.2.
7.2 Dental preparation
7.2.1 Cavity preparation
Remove all tartar and soft dirt from the tooth surface. Clean and disinfect the tooth surface with 3% (volume fraction) hydrogen peroxide, then use iodine or wash
Bite disinfectant disinfection. Prepare the required number of V-type holes on the buccal or labial side of the teeth with a sharp burr with sufficient water vapor spray. Place
The residual dentin thickness at the bottom of the cavity is less than 1 mm, preferably less than 0.5 mm, but does not expose the pulp. Except for filling test materials, different requirements
In addition to the operation, the water is used to clean the cavity and the cavity is dried with absorbent cotton.
Note 1. If the animal has obvious inflammation of the gums, the calculus and soft scale should be removed a few days before the cavity is prepared, and even the cleaning should be repeated until the gum inflammation is obtained.
control.
Note 2. In the process of cavity preparation, a calibrated impedance measuring instrument, such as a apex instrument, can be used to assist in estimating the residual dentin thickness.
7.2.2 Preparation of test materials
Prepare the test materials according to the product instructions. If the product specification requires the use of a liner material or a cavity treatment agent (such as dentin bonding)
(agent), then add these operations according to the manufacturer's instructions.
7.2.3 Cavity filling
According to the principle of random distribution, at least 7 holes were filled with test materials for each test period, and 4 nests were filled with negative control materials.
hole. If necessary (see note), there should be 4 positive control sockets per test cycle.
Note. Laboratories with a previous positive control database do not have to do a positive control test unless a positive response is sometimes required.
7.2.4 Postoperative observation and care
It is recommended to observe the animal status at least once a day after surgery and record abnormal performance. Take appropriate measures to change postoperative dietary habits, inflammation
The pain or pain caused by the disease or infection is minimized. Analgesics are given as needed after surgery.
7.3 Slice preparation
7.3.1 Drawing and fixing
Sufficient animals were sacrificed with excess anesthetics or other widely accepted substances at 7d ± 2d, 28d ± 3d and 70d ± 5d
At least 7 teeth in each test cycle were tested with test materials. Check the filling, teeth and supporting tissues to record any anomalies. Take off
Each tissue block containing the treated tooth and its surrounding soft and hard support tissue is fixed with a suitable fixative.
Note. Before the animals are sacrificed, the tissue infusion is performed with a fixative before the tissue block is removed, and the fixation effect is better.
7.3.2 Slicing and dyeing
After fixation, the teeth are decalcified with a suitable decalcifying agent [eg 10% (volume fraction) formic acid or 0.5 mol/LEDTA solution at pH 7.4].
Serial sections were prepared. The teeth are sliced along the long axis of the tooth through the cavity of the tooth, and the thickness is 5 μm to 7 μm. Hematoxylin-eosin (HE) staining, necessary
The stains were taken at intervals using appropriate bacterial staining methods (such as Brown and Brenn) or other methods for testing bacteria.
7.4 Dentin and pulp evaluation
The slice is blindly examined (not known as a test sample or a control sample). For each serial section, comprehensively describe and record dentin, pulp
And all histological features of the periapical tissue, including any histological changes that may be caused by cavity preparation. From serial slices, through
At least 5 sections of the fossa are equally spaced to analyze the inflammatory condition. According to Table 1, the surface pulp tissue (including the odontoblast layer, no fine
The inflammatory infiltration of the cell area and the rich cell area) and the remaining pulp tissue (deep tissue) were graded.
Table 1 Classification criteria for dental pulp dentin application test
Inflammation graded inflammation change description
0 No inflammation. the structure of the pulp tissue adjacent to the bottom of the cavity through the dentinal tubule is normal.
1 mild inflammation. the pulp tissue adjacent to the bottom of the cavity through the dentin tubules is normal, scattered inflammatory cells
2 Moderate inflammation. The normal structure of the pulp tissue adjacent to the bottom of the dentin is still present, and there is a small inflammatory cell aggregation lesion.
3 Severe inflammation. loss of pulp tissue structure through the dentinal tubule and the bottom of the cavity, extensive infiltration of inflammatory cells
4 Abscess formation or extensive inflammatory cell infiltration into tissue regions other than pulp tissue adjacent to the dentinal tubules
When evaluating each slice, record the minimum thickness of the remaining dentin, including measuring the bottom of the hole from the vertical angle to the endodontic - (pre-) dentin boundary
The distance of the face and the distance measured along the direction of the dentinal tubules. The latter means that when the slice plane and the dentinal tubules travel incompletely
When the time comes, that each dentinal tubule in the area fails to extend from the bottom of the hole to the endodontic- (pre-) dentin interface, it should follow the dentinal tubule
The general direction of the line is measured. Calculate the inflammatory response index for each test period, ie, add the scores of the grades obtained for each slice.
Take the total number of slices observed.
The test data shall be separately listed for the test materials (including the recommended liner material and cavity treatment agent), the negative control and the positive pair.
The result of the photo. For the cavity filled with the positive control material, the data previously used under the same test conditions can be used. In addition, record each test
The periodic test material and the control material...
Share