1
/
of
11
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 0454-2008 English PDF (YYT0454-2008)
YY/T 0454-2008 English PDF (YYT0454-2008)
Regular price
$230.00 USD
Regular price
Sale price
$230.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get QUOTATION in 1-minute: Click YY/T 0454-2008
Historical versions: YY/T 0454-2008
Preview True-PDF (Reload/Scroll if blank)
YY/T 0454-2008: Disposable scalpel
YY/T 0454-2008
PHARMACEUTICALS INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.30
C 31
Replacing YY/T 0454-2003
Disposable scalpel
ISSUED ON: OCTOBER 17, 2008
IMPLEMENTED ON: JANUARY 01, 2010
Issued by: China Food and Drug Administration
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative references ... 4
3 Structure and marking ... 5
4 Requirements ... 5
5 Test method ... 6
6 Inspection rules ... 6
7 Sign ... 8
8 Packing, transportation, storage, use period ... 8
Appendix A (Normative) Test of connection firmness between blade and handle ... 10
References ... 11
Disposable scalpel
1 Scope
This standard specifies the structure and marking, requirements, test methods,
inspection rules, signs, packaging, transportation, storage, use period of disposable
scalpels.
This standard applies to disposable scalpels (hereinafter referred to as scalpels), in
sterile packaging for single use, which are used to cut soft tissue, during surgery or
dissection.
2 Normative references
The provisions in following documents become the provisions of this Standard through
reference in this Standard. For the dated references, the subsequent amendments
(excluding corrections) or revisions do not apply to this Standard; however, parties who
reach an agreement based on this Standard are encouraged to study if the latest versions
of these documents are applicable. For undated references, the latest edition of the
referenced document applies.
GB/T 1220-2007 Stainless steel bars
GB/T 1298-1986 Carbon tool steels - Technical requirements
GB/T 1299-2000 Alloy tool steels
GB/T 2828.1-2003 Sampling procedures for inspection by attributes - Part 1:
Sampling schemes indexed by acceptance quality limit (AQL) for lot-by-lot
inspection (ISO 2859-1:1999, IDT)
GB/T 16886.7-2001 Biological evaluation of medical devices - Part 7: Ethylene
oxide sterilization residuals (idt ISO 10993-7:1995)
YY/T 0171-2008 Surgical instruments - Packaging, marking and instructions
YY 0174-2005 Scalpel blade
YY/T 0466-2003 Medical devices - Symbols to be used with medical device labels,
labelling and information to be supplied (ISO 15223:2000, IDT)
Pharmacopoeia of the People's Republic of China, 2005 edition, Part 2
4.4 The surgical blade, which is connected with the plastic handle, shall comply with
the provisions of 4.2, 4.3, 4.4 in YY 0174-2005.
4.5 The connection between the scalpel blade and the handle shall be firm.
4.6 The scalpel shall be provided with the cutting-edge protection device.
4.7 The sterilization process, for which the scalpel has been confirmed, shall be sterile.
4.8 When using ethylene oxide for sterilization, the residual amount of ethylene oxide
shall not exceed 10 μg/g.
5 Test method
5.1 Appearance test
Carry out visual observation. It shall comply with the provisions of 4.1, 4.2, 4.3, 4.6.
5.2 Scalpel blade test
Carry out the test according to the provisions of 5.2, 5.3, 5.4 in YY 0174-2005. The
results shall meet the provisions of 4.4.
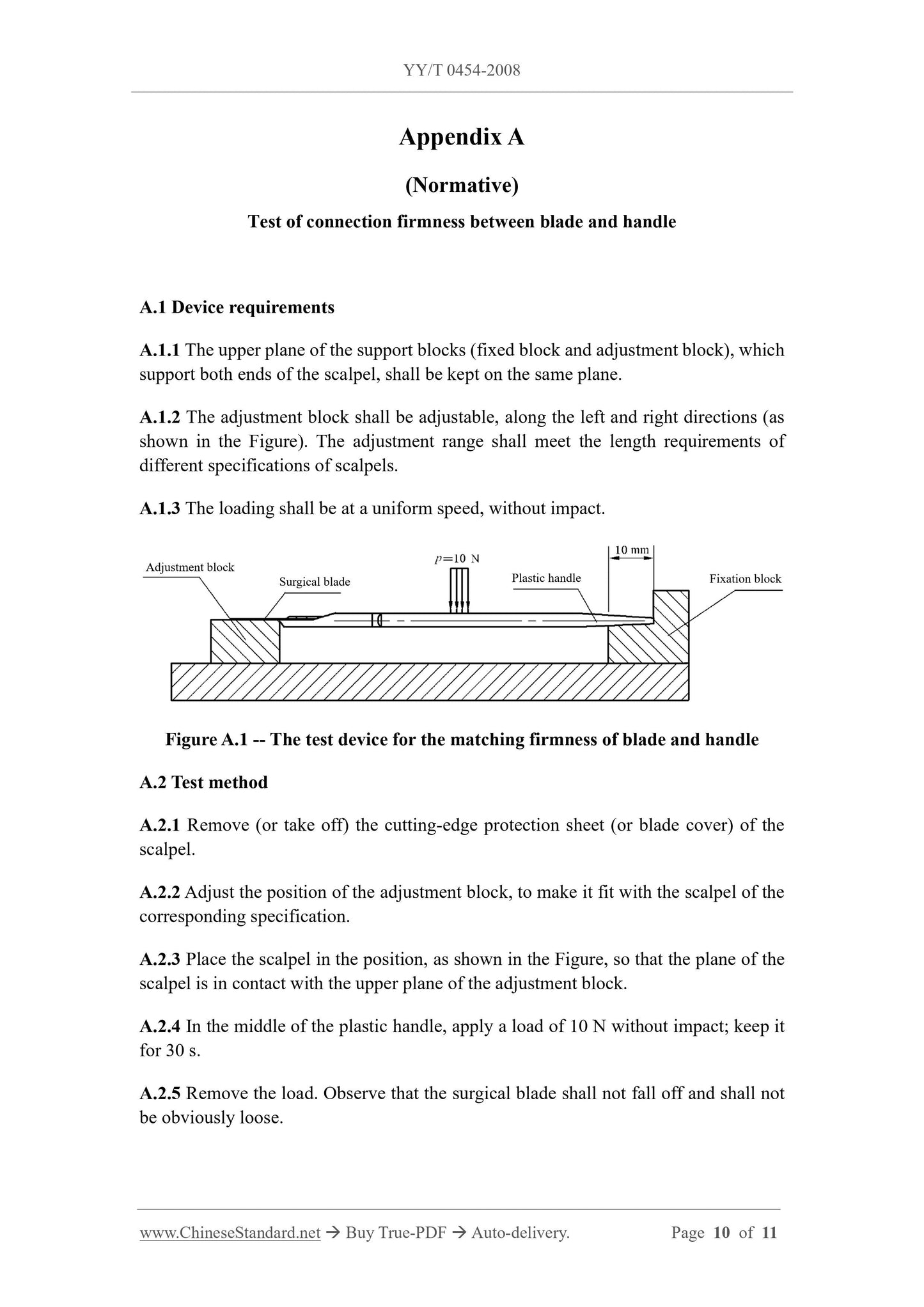
5.3 Connection firmness test
Carry out the test, according to the test method of Appendix A. The results shall meet
the requirements of 4.5.
5.4 Sterility inspection
Randomly select 10 scalpels, from each sterilized batch. Use the aseptic method, which
is specified in the "Pharmacopoeia of the People's Republic of China, 2005 edition Part
2". The results shall meet the requirements of 4.7.
5.5 Residual amount of ethylene oxide
It is carried out, according to the "Gas chromatography" in GB/T 16886.7-2001. It shall
comply with the provisions of 4.8.
6 Inspection rules
6.1 Acceptance
The scalpel shall be inspected by the technical inspection department of the
manufacturer. It can be submitted for acceptance, only after passing the inspection.
6.2 Inspection method
6.4.3 Judgment rules for periodic inspection: If all the test items in the periodic
inspection are qualified, the current periodic inspection is determined to be qualified;
otherwise, the periodic inspection is determined to be unqualified.
7 Sign
7.1 Product sign
At an appropriate part of each scalpel (or its small package), it shall be marked with the
model, which is specified in 3.2.
7.2 Packaging sign
7.2.1 The packaging sign shall comply with the provisions of 4.1.1, 4.1.3, 4.2.1, 4.2.3
in YY/T 0171-2008.
7.2.2 The certificate of conformity shall comply with the provisions in 4.3 of YY/T
0171-2008.
7.2.3 The signs and/or symbols, on the package, shall comply with the relevant
provisions in YY 0466.
8 Packing, transportation, storage, use period
8.1 Packaging
8.1.1 Each scalpel shall be packaged in an independent, sealed bag.
8.1.2 The packaging of each scalpel shall be able to ensure the sterility of the product
within the period of use, meanwhile ensure that the blade does not rust.
8.1.3 A certain number of small packaged products consist of medium packaging, which
is the smallest packaging unit for inspection and sales.
8.1.4 The large package shall have sufficient strength, to ensure that the product will
not be damaged, under normal transportation and storage conditions. The words and
graphic signs, on the large package, shall be guaranteed not to be blurred, due to a long
period of time.
The packaging of special requirements shall be carried out, according to the provisions
of the order contract.
8.2 Transportation
Transportation is carried out, according to the provisions of the order contract.
Get QUOTATION in 1-minute: Click YY/T 0454-2008
Historical versions: YY/T 0454-2008
Preview True-PDF (Reload/Scroll if blank)
YY/T 0454-2008: Disposable scalpel
YY/T 0454-2008
PHARMACEUTICALS INDUSTRY STANDARD
OF THE PEOPLE’S REPUBLIC OF CHINA
ICS 11.040.30
C 31
Replacing YY/T 0454-2003
Disposable scalpel
ISSUED ON: OCTOBER 17, 2008
IMPLEMENTED ON: JANUARY 01, 2010
Issued by: China Food and Drug Administration
Table of Contents
Foreword ... 3
1 Scope ... 4
2 Normative references ... 4
3 Structure and marking ... 5
4 Requirements ... 5
5 Test method ... 6
6 Inspection rules ... 6
7 Sign ... 8
8 Packing, transportation, storage, use period ... 8
Appendix A (Normative) Test of connection firmness between blade and handle ... 10
References ... 11
Disposable scalpel
1 Scope
This standard specifies the structure and marking, requirements, test methods,
inspection rules, signs, packaging, transportation, storage, use period of disposable
scalpels.
This standard applies to disposable scalpels (hereinafter referred to as scalpels), in
sterile packaging for single use, which are used to cut soft tissue, during surgery or
dissection.
2 Normative references
The provisions in following documents become the provisions of this Standard through
reference in this Standard. For the dated references, the subsequent amendments
(excluding corrections) or revisions do not apply to this Standard; however, parties who
reach an agreement based on this Standard are encouraged to study if the latest versions
of these documents are applicable. For undated references, the latest edition of the
referenced document applies.
GB/T 1220-2007 Stainless steel bars
GB/T 1298-1986 Carbon tool steels - Technical requirements
GB/T 1299-2000 Alloy tool steels
GB/T 2828.1-2003 Sampling procedures for inspection by attributes - Part 1:
Sampling schemes indexed by acceptance quality limit (AQL) for lot-by-lot
inspection (ISO 2859-1:1999, IDT)
GB/T 16886.7-2001 Biological evaluation of medical devices - Part 7: Ethylene
oxide sterilization residuals (idt ISO 10993-7:1995)
YY/T 0171-2008 Surgical instruments - Packaging, marking and instructions
YY 0174-2005 Scalpel blade
YY/T 0466-2003 Medical devices - Symbols to be used with medical device labels,
labelling and information to be supplied (ISO 15223:2000, IDT)
Pharmacopoeia of the People's Republic of China, 2005 edition, Part 2
4.4 The surgical blade, which is connected with the plastic handle, shall comply with
the provisions of 4.2, 4.3, 4.4 in YY 0174-2005.
4.5 The connection between the scalpel blade and the handle shall be firm.
4.6 The scalpel shall be provided with the cutting-edge protection device.
4.7 The sterilization process, for which the scalpel has been confirmed, shall be sterile.
4.8 When using ethylene oxide for sterilization, the residual amount of ethylene oxide
shall not exceed 10 μg/g.
5 Test method
5.1 Appearance test
Carry out visual observation. It shall comply with the provisions of 4.1, 4.2, 4.3, 4.6.
5.2 Scalpel blade test
Carry out the test according to the provisions of 5.2, 5.3, 5.4 in YY 0174-2005. The
results shall meet the provisions of 4.4.
5.3 Connection firmness test
Carry out the test, according to the test method of Appendix A. The results shall meet
the requirements of 4.5.
5.4 Sterility inspection
Randomly select 10 scalpels, from each sterilized batch. Use the aseptic method, which
is specified in the "Pharmacopoeia of the People's Republic of China, 2005 edition Part
2". The results shall meet the requirements of 4.7.
5.5 Residual amount of ethylene oxide
It is carried out, according to the "Gas chromatography" in GB/T 16886.7-2001. It shall
comply with the provisions of 4.8.
6 Inspection rules
6.1 Acceptance
The scalpel shall be inspected by the technical inspection department of the
manufacturer. It can be submitted for acceptance, only after passing the inspection.
6.2 Inspection method
6.4.3 Judgment rules for periodic inspection: If all the test items in the periodic
inspection are qualified, the current periodic inspection is determined to be qualified;
otherwise, the periodic inspection is determined to be unqualified.
7 Sign
7.1 Product sign
At an appropriate part of each scalpel (or its small package), it shall be marked with the
model, which is specified in 3.2.
7.2 Packaging sign
7.2.1 The packaging sign shall comply with the provisions of 4.1.1, 4.1.3, 4.2.1, 4.2.3
in YY/T 0171-2008.
7.2.2 The certificate of conformity shall comply with the provisions in 4.3 of YY/T
0171-2008.
7.2.3 The signs and/or symbols, on the package, shall comply with the relevant
provisions in YY 0466.
8 Packing, transportation, storage, use period
8.1 Packaging
8.1.1 Each scalpel shall be packaged in an independent, sealed bag.
8.1.2 The packaging of each scalpel shall be able to ensure the sterility of the product
within the period of use, meanwhile ensure that the blade does not rust.
8.1.3 A certain number of small packaged products consist of medium packaging, which
is the smallest packaging unit for inspection and sales.
8.1.4 The large package shall have sufficient strength, to ensure that the product will
not be damaged, under normal transportation and storage conditions. The words and
graphic signs, on the large package, shall be guaranteed not to be blurred, due to a long
period of time.
The packaging of special requirements shall be carried out, according to the provisions
of the order contract.
8.2 Transportation
Transportation is carried out, according to the provisions of the order contract.
Share