1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 0659-2017 English PDF (YY/T0659-2017)
YY/T 0659-2017 English PDF (YY/T0659-2017)
Regular price
$150.00 USD
Regular price
Sale price
$150.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY/T 0659-2017 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 0659-2017
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 0659-2017: Blood coagulation analyzer
YY/T 0659-2017
Blood coagulation analyzer
ICS 11.100
C44
People's Republic of China Pharmaceutical Industry Standard
Replacing YY/T 0658-2008, YY/T 0659-2008
Coagulation analyzer
Released on.2017-03-28
2018-04-01 implementation
State Food and Drug Administration issued
Foreword
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
This standard integrates YY/T 0658-2008 "semi-automatic coagulation analyzer", YY/T 0659-2008 "automatic coagulation analyzer",
Compared with YY/T 0658-2008 and YY/T 0659-2008, the main changes except editorial changes are as follows.
--- Modified the standard name, "semi-automatic coagulation analyzer", "automatic coagulation analyzer" modified to "coagulation analyzer";
--- Revised the scope, increased platelet aggregation function and blood rheology function detection, immediate detection (POCT) instruments are not applicable to this standard
Explain the addition of instructions for instruments that are only suitable for coagulation testing (see Chapter 1);
--- The description of the text in the normative reference document is written in accordance with GB/T 1.1-2009;
--- Normative references are not dated, that is, the latest version applies to this standard;
--- Accuracy, precision, linearity, carrying pollution terms and definitions refer to the definition of generic terms already listed in GB/T 29791.1 (see
Chapter 3);
--- Revised the requirements of the sample in the precision project, increased the normal sample requirements, and deleted the abnormal sample requirements (more than 2 times higher than the normal sample)
Value) (see 5.7);
--- Modified the linear index r value requirement, r ≥ 0.980 (see 5.9);
--- Increased linear deviation requirements (see 5.9);
--- Revised the requirement of continuous working time, the continuous working time was changed from 24h to 8h, and the modification was required (see 5.10);
--- Added GB 4793.9, YY 0648 security requirements (see 5.13);
--- Added GB/T 18268.1, GB/T 18268.26 electromagnetic compatibility requirements (see 5.14);
--- Revised the test method for carrying pollution rate (see 6.6);
--- Modified the FIB accuracy test method to modify (see 6.9).
Please note that some of the contents of this document may involve patents. The issuing organization of this document is not responsible for identifying these patents.
This standard was proposed by the State Food and Drug Administration.
This standard is under the jurisdiction of the National Medical Clinical Laboratory and the In vitro Diagnostic System Standardization Technical Committee (SAC/TC136).
This standard was drafted. Beijing Medical Device Inspection Institute, Beijing Medical Device Technology Evaluation Center, and Xisen Meikang Medical Electronics (on
Hai) Co., Ltd., Beijing Seckey Technology Development Co., Ltd., Beijing Zhongqin Shidi Biotechnology Co., Ltd., Beijing Pulisheng Instrument Co., Ltd.
Company, Wolfen Medical Devices Trading Co., Ltd.
The main drafters of this standard. Xu Yong, Sun Wei, Su Jing, Song Wei, Ding Zhonghui, Li Gang, Zhang Yaohui, Jin Yan.
This standard replaces YY/T 0658-2008 and YY/T 0659-2008.
Coagulation analyzer
1 Scope
This standard specifies the terms and definitions of the coagulation analyzer, product classification, technical requirements, test methods, labels, markings and instructions for use, and packages.
Loading, transport and storage.
This standard is applicable to coagulation analyzers for clinical analysis of blood coagulation and anticoagulation, fibrinolysis and antifibrinolytic functions in patients.
This standard does not apply to instruments for platelet aggregation and hemorheological function testing, and immediate detection (POCT).
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article.
Pieces. For undated references, the latest edition (including all amendments) applies to this document.
GB/T 191 packaging storage and transportation icon
Safety of electrical equipment for measurement, control and laboratory use - Part 1. General requirements
GB 4793.9 Safety requirements for electrical equipment for measurement, control and laboratory use - Part 9. Laboratory analysis and other purposes
Special requirements for moving and semi-automatic equipment
GB/T 14710 Medical electrical requirements and test methods
GB/T 18268.1 Electromagnetic compatibility requirements for electrical equipment - Part 1 . General requirements
GB/T 18268.26 Electromagnetic compatibility requirements for electrical equipment for measurement, control and laboratory - Part 26. Particular requirements
External diagnostic (IVD) medical equipment
GB/T 29791.1 Information provided by in vitro diagnostic medical device manufacturers (labeling) Part 1. Terminology, definitions and general
Claim
GB/T 29791.3 Information provided by in vitro diagnostic medical device manufacturers (labeling) Part 3. Professional in vitro diagnostic equipment
Safety of electrical equipment for measurement, control and laboratory use - Part 2-101. In vitro diagnostic (IVD) medical equipment
Special requirements
3 Terms and definitions
The following terms and definitions apply to this document.
3.1
Semi-automatic semi-automated
Some analysis steps of the instrument or test system are mechanized, and other steps still require operator involvement.
3.2
Fully automatic ful-automated
All analytical steps of the instrument or test system are mechanized, including sample and reagent addition, sample/reagent interaction, and
Learning/biological analysis, result calculation and result reading.
3.3
Channel channel
In a measurement cycle, the reaction system can be detected and the detection results can be obtained.
3.4
Test speed throughputrate
The number of tests completed per unit time under specified conditions is usually expressed as "tests/hour". The test speed is related to the test project.
3.5
Carrying pollution rate carry-overrate
The specific quantitative indicators of carrying pollution reflect the impact of one sample on the performance of the next sample.
Note. The carrying rate is related to the specific test method.
3.6
Coagulation method
Simulate blood coagulation conditions, add a reagent, activate the blood coagulation effect, and convert fibrinogen in the sample into cross-linked fiber egg
White, causing the sample to solidify. By continuously monitoring the optics (eg absorbance), physics (eg viscosity) of the reaction system in this process
Or electrical (eg, current) characteristic changes determine the endpoint of the reaction, and as a transformation time of fibrinogen, use this principle to determine blood samples
A method of setting properties or fibrinolytic properties.
3.7
Accuracy
The degree to which a measured quantity is consistent with a measured true value.
[GB/T 29791.1-2013 definition A.3.24]
3.8
Precision precision
Under the specified conditions, the same or similar objects are repeatedly measured to obtain the degree of agreement between the measured values or the measured values.
[GB/T 29791.1-2013 definition A.3.29]
3.9
Linear linearity
The ability to measure the magnitude directly proportional to the measured value in the sample is given.
[GB/T 29791.1-2013 definition A.3.21]
3.10
Carrying pollution carryover
The introduction of a material that does not belong to it in the reaction mixture.
[GB/T 29791.1-2013 definition A.3.8]
4 Product Categories
4.1 Classification of automation
Semi-automated, fully automated.
4.2 Channel Type Classification (semi-automatic)
Single, dual and multi-channel.
5 Technical requirements
5.1 Preheating time
The preheating time should be less than 30 minutes.
5.2 Temperature control
5.2.1 The temperature of the reaction system of the detection unit and the incubator thermostat unit is controlled within the range of 37.0 °C ± 1.0 °C.
5.2.2 Reagent cooling temperature should be no higher than 20 °C.
5.3 Test items and reporting units
The test should include at least plasma prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen
(FIB), thrombin time (TT) assay. The reporting unit of PT, APTT, and TT is seconds (s), and the measurement result of PT should also be reported to the country.
The normalized ratio (INR), the reporting unit of FIB is g/L or mg/dL, and the reporting unit of clotting factor activity (automatic analyzer) is
U/L or percentage (%).
5.4 Channel difference (for semi-automatic analyzers)
The results from different channel tests are extremely poor ≤10%.
5.5 Carrying pollution rate (applicable to fully automatic analyzer)
5.5.1 Carrying pollution rate of sample concentration. FIB (g/L) carrying pollution rate should be ≤10%.
5.5.2 The carrying rate of FIB or TT on PT or APTT is in line with the manufacturer's nominal level.
5.6 Test speed
The test speed or constant test speed should not be less than the nominal test speed of the instrument manual.
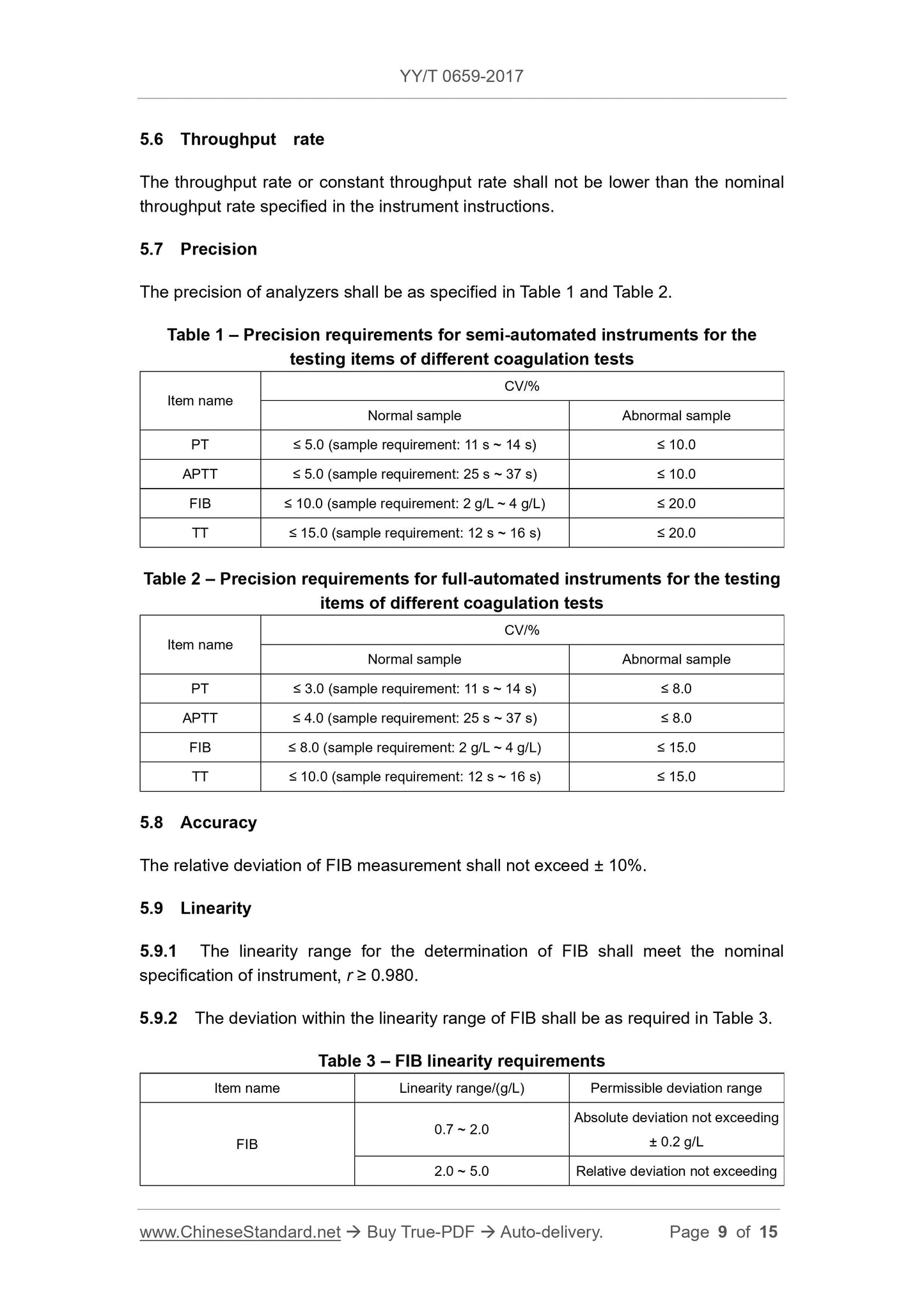
5.7 Precision
The precision of the analyzer should meet the requirements of Tables 1 and 2.
Table 1 Precision requirements for different coagulation test items for semi-automatic instruments
project name
CV/%
Normal sample abnormal sample
PT ≤ 5.0 (sample requirement. 11s~14s) ≤10.0
APTT ≤ 5.0 (sample requirement. 25s~37s) ≤10.0
FIB ≤10.0 (sample requirement. 2g/L~4g/L) ≤20.0
TT ≤15.0 (sample requirement. 12s~16s) ≤20.0
Table 2 Precision requirements for different coagulation test determination items of fully automatic instruments
project name
CV/%
Normal sample abnormal sample
PT ≤ 3.0 (sample requirements. 11s~14s) ≤8.0
APTT ≤ 4.0 (sample requirement. 25s~37s) ≤8.0
FIB ≤ 8.0 (sample requirements. 2g/L ~ 4g/L) ≤ 15.0
TT ≤ 10.0 (sample requirement. 12s~16s) ≤15.0
5.8 Accuracy
The relative deviation of FIB measurements does not exceed ±10%.
5.9 linear
5.9.1 The linear range of the FIB shall be determined to meet the nominal requirements of the instrument, r ≥ 0.980.
5.9.2 The deviation of the linear range of the FIB shall comply with the requirements of Table 3.
Table 3 Linear requirements of FIB
Project name linear range/(g/L) allowable deviation range
FIB
0.7~2.0 absolute deviation does not exceed ±0.2g/L
2.0~5.0 relative deviation does not exceed ±10%
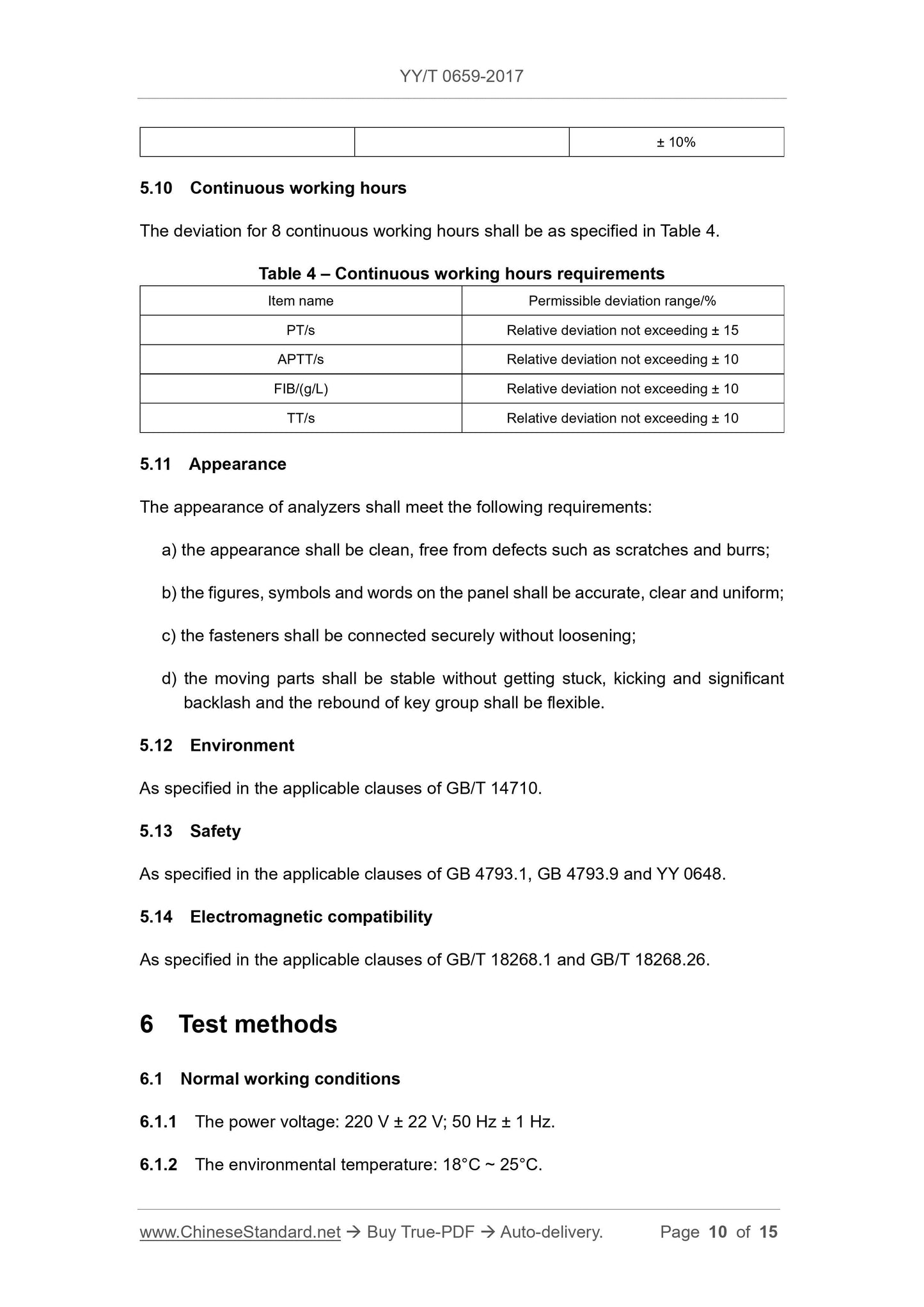
5.10 continuous working hours
The deviation of 8 hours of continuous operation shall comply with the requirements of Table 4.
Table 4 Continuous working time requirements
Project name allowable deviation range /%
PT/s relative deviation does not exceed ±15
APTT/s relative deviation does not exceed ±10
FIB/(g/L) relative deviation does not exceed ±10
TT/s relative deviation does not exceed ±10
5.11 Appearance
The appearance of the analyzer should meet the following requirements.
a) The appearance should be clean, free of scratches, burrs and other defects.
b) The graphics, symbols and text on the panel should be accurate, clear and uniform.
c) The fastener connection should be firm and reliable and must not be loose.
d) The moving parts should be stable, there should be no jamming, sudden jump and significant empty return, and the key group rebound should be flexible.
5.12 Environment
Should comply with the provisions of the applicable provisions of GB/T 14710.
5.13 Security
It shall comply with the applicable provisions of GB 4793.1, GB 4793.9 and YY 0648.
5.14 Electromagnetic compatibility
Should comply with the provisions of the applicable provisions of GB/T 18268.1, GB/T 18268.26.
6 Test methods
6.1 Normal working conditions
6.1.1 Power supply voltage. 220V ± 22V; 50Hz ± 1Hz.
6.1.2 Ambient temperature. 18 ° C ~ 25 ° C.
6.1.3 Relative humidity. ≤80%.
6.1.4 Atmospheric pressure. 86.0kPa~106.0kPa.
Note. When the conditions in 6.1.1~6.1.4 are inconsistent with the conditions stated in the manufacturing mark, the conditions specified in the product shall prevail.
6.2 Warming time
After the boot, follow the manufacturer's requirements for testing.
6.3 Temperature control
Test according to the method provided by the manufacturer.
6.4 Test items and reporting units
According to the instrument manual, the parameters of each item set by the instrument are retrieved for verification. Instruments and supporting reagents and blood can be used if necessary
The slurry sample was tested and verified.
6.5 channel difference (for semi-automatic analyzers)
Under normal conditions, the same normal specimens PT, APTT, TT, FIB were measured three times in different channels. Calculate each channel separately
The arithmetic mean (Xi) of the measured values and the total arithmetic mean (X total) of the measured values of all the channels, and then calculate the channel difference (R) according to the equation (1).
R=
(Xmax-Xmin)
X total
×100% (1)
In the formula.
R --- channel difference;
Xmax---the maximum of the arithmetic mean of the measured values of each channel;
Xmin---the minimum of the arithmetic mean of the measured values of each channel;
X total -- the total arithmetic mean of all channel measurements.
6.6 Carrying pollution rate (for fully automatic analyzer)
6.6.1 Carrying contamination rate of sample concentration
Take a high-concentration clinical sample, mix it evenly and measure it 3 times continuously, then take a low-concentration clinical sample, mix evenly and then
Continue to measure 3 times and calculate the carrying pollution rate according to formula (2).
CR=
|L1-L3|
H3-L3 ×
100% (2)
In the formula.
CR---carrying pollution rate;
L1 --- the first measurement of the low concentration clinical sample;
L3 --- the third measured value of the low concentration clinical sample;
H3---The third measured value of the high concentration clinical sample.
Note. The measured value of the high concentration sample should be greater than twice the measured value of the low concentration sample.
6.6.2 Carrying pollution rate of PT or APTT by FIB or TT
After continuous measurement of normal plasma PT or APTT three times ...
Get Quotation: Click YY/T 0659-2017 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 0659-2017
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 0659-2017: Blood coagulation analyzer
YY/T 0659-2017
Blood coagulation analyzer
ICS 11.100
C44
People's Republic of China Pharmaceutical Industry Standard
Replacing YY/T 0658-2008, YY/T 0659-2008
Coagulation analyzer
Released on.2017-03-28
2018-04-01 implementation
State Food and Drug Administration issued
Foreword
This standard was drafted in accordance with the rules given in GB/T 1.1-2009.
This standard integrates YY/T 0658-2008 "semi-automatic coagulation analyzer", YY/T 0659-2008 "automatic coagulation analyzer",
Compared with YY/T 0658-2008 and YY/T 0659-2008, the main changes except editorial changes are as follows.
--- Modified the standard name, "semi-automatic coagulation analyzer", "automatic coagulation analyzer" modified to "coagulation analyzer";
--- Revised the scope, increased platelet aggregation function and blood rheology function detection, immediate detection (POCT) instruments are not applicable to this standard
Explain the addition of instructions for instruments that are only suitable for coagulation testing (see Chapter 1);
--- The description of the text in the normative reference document is written in accordance with GB/T 1.1-2009;
--- Normative references are not dated, that is, the latest version applies to this standard;
--- Accuracy, precision, linearity, carrying pollution terms and definitions refer to the definition of generic terms already listed in GB/T 29791.1 (see
Chapter 3);
--- Revised the requirements of the sample in the precision project, increased the normal sample requirements, and deleted the abnormal sample requirements (more than 2 times higher than the normal sample)
Value) (see 5.7);
--- Modified the linear index r value requirement, r ≥ 0.980 (see 5.9);
--- Increased linear deviation requirements (see 5.9);
--- Revised the requirement of continuous working time, the continuous working time was changed from 24h to 8h, and the modification was required (see 5.10);
--- Added GB 4793.9, YY 0648 security requirements (see 5.13);
--- Added GB/T 18268.1, GB/T 18268.26 electromagnetic compatibility requirements (see 5.14);
--- Revised the test method for carrying pollution rate (see 6.6);
--- Modified the FIB accuracy test method to modify (see 6.9).
Please note that some of the contents of this document may involve patents. The issuing organization of this document is not responsible for identifying these patents.
This standard was proposed by the State Food and Drug Administration.
This standard is under the jurisdiction of the National Medical Clinical Laboratory and the In vitro Diagnostic System Standardization Technical Committee (SAC/TC136).
This standard was drafted. Beijing Medical Device Inspection Institute, Beijing Medical Device Technology Evaluation Center, and Xisen Meikang Medical Electronics (on
Hai) Co., Ltd., Beijing Seckey Technology Development Co., Ltd., Beijing Zhongqin Shidi Biotechnology Co., Ltd., Beijing Pulisheng Instrument Co., Ltd.
Company, Wolfen Medical Devices Trading Co., Ltd.
The main drafters of this standard. Xu Yong, Sun Wei, Su Jing, Song Wei, Ding Zhonghui, Li Gang, Zhang Yaohui, Jin Yan.
This standard replaces YY/T 0658-2008 and YY/T 0659-2008.
Coagulation analyzer
1 Scope
This standard specifies the terms and definitions of the coagulation analyzer, product classification, technical requirements, test methods, labels, markings and instructions for use, and packages.
Loading, transport and storage.
This standard is applicable to coagulation analyzers for clinical analysis of blood coagulation and anticoagulation, fibrinolysis and antifibrinolytic functions in patients.
This standard does not apply to instruments for platelet aggregation and hemorheological function testing, and immediate detection (POCT).
2 Normative references
The following documents are indispensable for the application of this document. For dated references, only dated versions apply to this article.
Pieces. For undated references, the latest edition (including all amendments) applies to this document.
GB/T 191 packaging storage and transportation icon
Safety of electrical equipment for measurement, control and laboratory use - Part 1. General requirements
GB 4793.9 Safety requirements for electrical equipment for measurement, control and laboratory use - Part 9. Laboratory analysis and other purposes
Special requirements for moving and semi-automatic equipment
GB/T 14710 Medical electrical requirements and test methods
GB/T 18268.1 Electromagnetic compatibility requirements for electrical equipment - Part 1 . General requirements
GB/T 18268.26 Electromagnetic compatibility requirements for electrical equipment for measurement, control and laboratory - Part 26. Particular requirements
External diagnostic (IVD) medical equipment
GB/T 29791.1 Information provided by in vitro diagnostic medical device manufacturers (labeling) Part 1. Terminology, definitions and general
Claim
GB/T 29791.3 Information provided by in vitro diagnostic medical device manufacturers (labeling) Part 3. Professional in vitro diagnostic equipment
Safety of electrical equipment for measurement, control and laboratory use - Part 2-101. In vitro diagnostic (IVD) medical equipment
Special requirements
3 Terms and definitions
The following terms and definitions apply to this document.
3.1
Semi-automatic semi-automated
Some analysis steps of the instrument or test system are mechanized, and other steps still require operator involvement.
3.2
Fully automatic ful-automated
All analytical steps of the instrument or test system are mechanized, including sample and reagent addition, sample/reagent interaction, and
Learning/biological analysis, result calculation and result reading.
3.3
Channel channel
In a measurement cycle, the reaction system can be detected and the detection results can be obtained.
3.4
Test speed throughputrate
The number of tests completed per unit time under specified conditions is usually expressed as "tests/hour". The test speed is related to the test project.
3.5
Carrying pollution rate carry-overrate
The specific quantitative indicators of carrying pollution reflect the impact of one sample on the performance of the next sample.
Note. The carrying rate is related to the specific test method.
3.6
Coagulation method
Simulate blood coagulation conditions, add a reagent, activate the blood coagulation effect, and convert fibrinogen in the sample into cross-linked fiber egg
White, causing the sample to solidify. By continuously monitoring the optics (eg absorbance), physics (eg viscosity) of the reaction system in this process
Or electrical (eg, current) characteristic changes determine the endpoint of the reaction, and as a transformation time of fibrinogen, use this principle to determine blood samples
A method of setting properties or fibrinolytic properties.
3.7
Accuracy
The degree to which a measured quantity is consistent with a measured true value.
[GB/T 29791.1-2013 definition A.3.24]
3.8
Precision precision
Under the specified conditions, the same or similar objects are repeatedly measured to obtain the degree of agreement between the measured values or the measured values.
[GB/T 29791.1-2013 definition A.3.29]
3.9
Linear linearity
The ability to measure the magnitude directly proportional to the measured value in the sample is given.
[GB/T 29791.1-2013 definition A.3.21]
3.10
Carrying pollution carryover
The introduction of a material that does not belong to it in the reaction mixture.
[GB/T 29791.1-2013 definition A.3.8]
4 Product Categories
4.1 Classification of automation
Semi-automated, fully automated.
4.2 Channel Type Classification (semi-automatic)
Single, dual and multi-channel.
5 Technical requirements
5.1 Preheating time
The preheating time should be less than 30 minutes.
5.2 Temperature control
5.2.1 The temperature of the reaction system of the detection unit and the incubator thermostat unit is controlled within the range of 37.0 °C ± 1.0 °C.
5.2.2 Reagent cooling temperature should be no higher than 20 °C.
5.3 Test items and reporting units
The test should include at least plasma prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen
(FIB), thrombin time (TT) assay. The reporting unit of PT, APTT, and TT is seconds (s), and the measurement result of PT should also be reported to the country.
The normalized ratio (INR), the reporting unit of FIB is g/L or mg/dL, and the reporting unit of clotting factor activity (automatic analyzer) is
U/L or percentage (%).
5.4 Channel difference (for semi-automatic analyzers)
The results from different channel tests are extremely poor ≤10%.
5.5 Carrying pollution rate (applicable to fully automatic analyzer)
5.5.1 Carrying pollution rate of sample concentration. FIB (g/L) carrying pollution rate should be ≤10%.
5.5.2 The carrying rate of FIB or TT on PT or APTT is in line with the manufacturer's nominal level.
5.6 Test speed
The test speed or constant test speed should not be less than the nominal test speed of the instrument manual.
5.7 Precision
The precision of the analyzer should meet the requirements of Tables 1 and 2.
Table 1 Precision requirements for different coagulation test items for semi-automatic instruments
project name
CV/%
Normal sample abnormal sample
PT ≤ 5.0 (sample requirement. 11s~14s) ≤10.0
APTT ≤ 5.0 (sample requirement. 25s~37s) ≤10.0
FIB ≤10.0 (sample requirement. 2g/L~4g/L) ≤20.0
TT ≤15.0 (sample requirement. 12s~16s) ≤20.0
Table 2 Precision requirements for different coagulation test determination items of fully automatic instruments
project name
CV/%
Normal sample abnormal sample
PT ≤ 3.0 (sample requirements. 11s~14s) ≤8.0
APTT ≤ 4.0 (sample requirement. 25s~37s) ≤8.0
FIB ≤ 8.0 (sample requirements. 2g/L ~ 4g/L) ≤ 15.0
TT ≤ 10.0 (sample requirement. 12s~16s) ≤15.0
5.8 Accuracy
The relative deviation of FIB measurements does not exceed ±10%.
5.9 linear
5.9.1 The linear range of the FIB shall be determined to meet the nominal requirements of the instrument, r ≥ 0.980.
5.9.2 The deviation of the linear range of the FIB shall comply with the requirements of Table 3.
Table 3 Linear requirements of FIB
Project name linear range/(g/L) allowable deviation range
FIB
0.7~2.0 absolute deviation does not exceed ±0.2g/L
2.0~5.0 relative deviation does not exceed ±10%
5.10 continuous working hours
The deviation of 8 hours of continuous operation shall comply with the requirements of Table 4.
Table 4 Continuous working time requirements
Project name allowable deviation range /%
PT/s relative deviation does not exceed ±15
APTT/s relative deviation does not exceed ±10
FIB/(g/L) relative deviation does not exceed ±10
TT/s relative deviation does not exceed ±10
5.11 Appearance
The appearance of the analyzer should meet the following requirements.
a) The appearance should be clean, free of scratches, burrs and other defects.
b) The graphics, symbols and text on the panel should be accurate, clear and uniform.
c) The fastener connection should be firm and reliable and must not be loose.
d) The moving parts should be stable, there should be no jamming, sudden jump and significant empty return, and the key group rebound should be flexible.
5.12 Environment
Should comply with the provisions of the applicable provisions of GB/T 14710.
5.13 Security
It shall comply with the applicable provisions of GB 4793.1, GB 4793.9 and YY 0648.
5.14 Electromagnetic compatibility
Should comply with the provisions of the applicable provisions of GB/T 18268.1, GB/T 18268.26.
6 Test methods
6.1 Normal working conditions
6.1.1 Power supply voltage. 220V ± 22V; 50Hz ± 1Hz.
6.1.2 Ambient temperature. 18 ° C ~ 25 ° C.
6.1.3 Relative humidity. ≤80%.
6.1.4 Atmospheric pressure. 86.0kPa~106.0kPa.
Note. When the conditions in 6.1.1~6.1.4 are inconsistent with the conditions stated in the manufacturing mark, the conditions specified in the product shall prevail.
6.2 Warming time
After the boot, follow the manufacturer's requirements for testing.
6.3 Temperature control
Test according to the method provided by the manufacturer.
6.4 Test items and reporting units
According to the instrument manual, the parameters of each item set by the instrument are retrieved for verification. Instruments and supporting reagents and blood can be used if necessary
The slurry sample was tested and verified.
6.5 channel difference (for semi-automatic analyzers)
Under normal conditions, the same normal specimens PT, APTT, TT, FIB were measured three times in different channels. Calculate each channel separately
The arithmetic mean (Xi) of the measured values and the total arithmetic mean (X total) of the measured values of all the channels, and then calculate the channel difference (R) according to the equation (1).
R=
(Xmax-Xmin)
X total
×100% (1)
In the formula.
R --- channel difference;
Xmax---the maximum of the arithmetic mean of the measured values of each channel;
Xmin---the minimum of the arithmetic mean of the measured values of each channel;
X total -- the total arithmetic mean of all channel measurements.
6.6 Carrying pollution rate (for fully automatic analyzer)
6.6.1 Carrying contamination rate of sample concentration
Take a high-concentration clinical sample, mix it evenly and measure it 3 times continuously, then take a low-concentration clinical sample, mix evenly and then
Continue to measure 3 times and calculate the carrying pollution rate according to formula (2).
CR=
|L1-L3|
H3-L3 ×
100% (2)
In the formula.
CR---carrying pollution rate;
L1 --- the first measurement of the low concentration clinical sample;
L3 --- the third measured value of the low concentration clinical sample;
H3---The third measured value of the high concentration clinical sample.
Note. The measured value of the high concentration sample should be greater than twice the measured value of the low concentration sample.
6.6.2 Carrying pollution rate of PT or APTT by FIB or TT
After continuous measurement of normal plasma PT or APTT three times ...
Share