1
/
of
9
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 0681.16-2019 English PDF (YY/T0681.16-2019)
YY/T 0681.16-2019 English PDF (YY/T0681.16-2019)
Regular price
$115.00 USD
Regular price
Sale price
$115.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY/T 0681.16-2019 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 0681.16-2019
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 0681.16-2019: Test methods for sterile medical device package - Part 16: Test for climatic stressing of packaging system
YY/T 0681.16-2019
Test methods for sterile medical device package-Part 16.Test for climatic stressing of packaging system
ICS 11.080.040
C31
People's Republic of China Pharmaceutical Industry Standard
Sterile medical device packaging test methods
Part 16.Climate Resilience Test of Packaging System
2019-05-31 released
2020-06-01 Implementation
Issued by the State Drug Administration
Preface
YY/T 0681 "Test Methods for Sterile Medical Device Packaging" consists of the following parts.
---Part 1.Guide to accelerated aging test;
---Part 2.Sealing strength of soft barrier materials;
---Part 3.Unconstrained packaging resists internal pressure damage;
---Part 4.Dyeing liquid penetration method to determine the seal leakage of breathable packaging;
---Part 5.Internal pressure method to detect gross leaks (bubble method);
---Part 6.Evaluation of chemical resistance of printing ink and coating on flexible packaging materials;
---Part 7.Use tape to evaluate the adhesion of ink or coating on flexible packaging materials;
---Part 8.Determination of the weight of the adhesive layer;
---Part 9.Seal burst test of flexible packaging by air pressure inside restraint plate;
---Part 10.Microbial barrier grading test for breathable packaging materials;
---Part 11.Visually inspect the seal integrity of medical packaging;
---Part 12.Anti-rubbing of soft barrier film;
---Part 13.Soft barrier film and composite film resistance to slow puncture;
---Part 14.Test of moisture and dry microbial barrier of breathable packaging materials;
---Part 15.Performance test of transport containers and systems;
---Part 16.Test of Climate Response Ability of Packaging System.
This part is part 16 of YY/T 0681.
This section was drafted in accordance with the rules given in GB/T 1.1-2009.
Please note that certain contents of this document may involve patents. The issuing agency of this document is not responsible for identifying these patents.
This part was proposed by the State Drug Administration.
This part is under the jurisdiction of the National Standardization Technical Committee for Medical Infusion Devices (SAC/TC106).
The main drafting units of this section. Shandong Medical Device Product Quality Inspection Center, Shandong Weigao Group Medical Polymer Products Co., Ltd.
Company, Shanghai MicroPort Medical Equipment (Group) Co., Ltd.
The main drafters of this section. Wang Dongwei, Li Weiyang, Li Yong, Dong Dandan.
Sterile medical device packaging test methods
Part 16.Climate Resilience Test of Packaging System
1 Scope
This part of YY/T 0681 specifies the climatic stress that the sterile medical device packaging system may bear during the circulation cycle in the laboratory.
It can still provide a unified benchmark for product protection from damage or change under the effect of change.
This part is applicable as an independent test to evaluate the climate resilience of the packaging system; it is also applicable to the packaging system as a single package.
The system is subjected to the state adjustment method before the test required for overnight or two-day supply.
This section does not apply to the climate resilience test of refrigerated, frozen or low-temperature storage and transportation packaging systems.
2 Terms and definitions
The following terms and definitions apply to this document.
2.1
Climate strain
Expose the test sample of the packaging system to temperature and humidity conditions for a specified period of time to simulate the expected storage and
Circulation system conditions.
3 overview
Devices that are transported in the circulation environment (regardless of the mode of transport) will be transported to various places (especially import and export) at various times during the year.
Various climates and physical environments. This part is designed to establish the climatic conditions and duration that may be encountered during transportation to the packaging system.
The climate change caused by the system provides guidance to evaluate the ability of packaging to protect the contents or devices during the circulation process.
4 Significance and application
This section provides methods for adjusting the state of the packaging system using the climatic conditions that occur in the actual circulation process. Recommended exposure
The level is based on information about existing transportation, handling and storage environments, current industrial regulations, and published research. These conditions
Not absolute extreme conditions, but they are the average of the daily records of cold and hot climates in the world.
5 Instruments
5.1 The room (or box), whose specifications enable a single sample container or package to be exposed to circulating air at the selected temperature and humidity.
5.2 The control instrument can maintain the indoor environment within the allowable limits of the required environmental conditions (see Table 1).
5.3 Hygrometer, an instrument used to indicate relative humidity, its relative humidity accuracy should be ±2%. The wet and dry bulb thermometer can directly measure the relative
Humidity can also be used to verify hygrometer.
5.4 Thermometers and temperature measuring equipment with an accuracy of 0.1℃ can be used. It can be directly measured with a dry bulb thermometer or a wet bulb thermometer
Or check the temperature indicator.
6 Sample
6.1 The sample should contain a representative packaging system, including the actual contents or devices. Can use defective or less defective interior
The premise is that the defect is not in the research scope and the defect is recorded in the report. Simulated contents can also be used, provided they are
The loading characteristics of the contents are the same.
6.2 Care should be taken to ensure that the packaging system and its contents do not degrade before being exposed to weather stress. This is for the need to send samples
It is especially important when going to a remote test site.
7 Procedure
7.1 Describe the packaging system according to specifications, weight and composition materials, and determine the test unit (shipping box, multiple packages, pallet goods, etc.).
7.2 Identify the various transportation modes and durations used for storage and the circulation of products to customers. For example, products will be shipped by land
And air freight is sent to the destination after a two-day shipping period.
7.3 When used in an independent test, the acceptance criteria are determined in advance.
7.4 Use the sequence, conditions and exposure time given in Table 1 to write a test plan. If the conditions and exposure of the user’s circulation environment are known
If the time is different from that specified in Table 1, the user’s circulation conditions should be used.
Note. Exposing packaging system materials to hot/humid climate strains may cause the materials to absorb moisture, which may remain in the remaining exposure process
In the material.
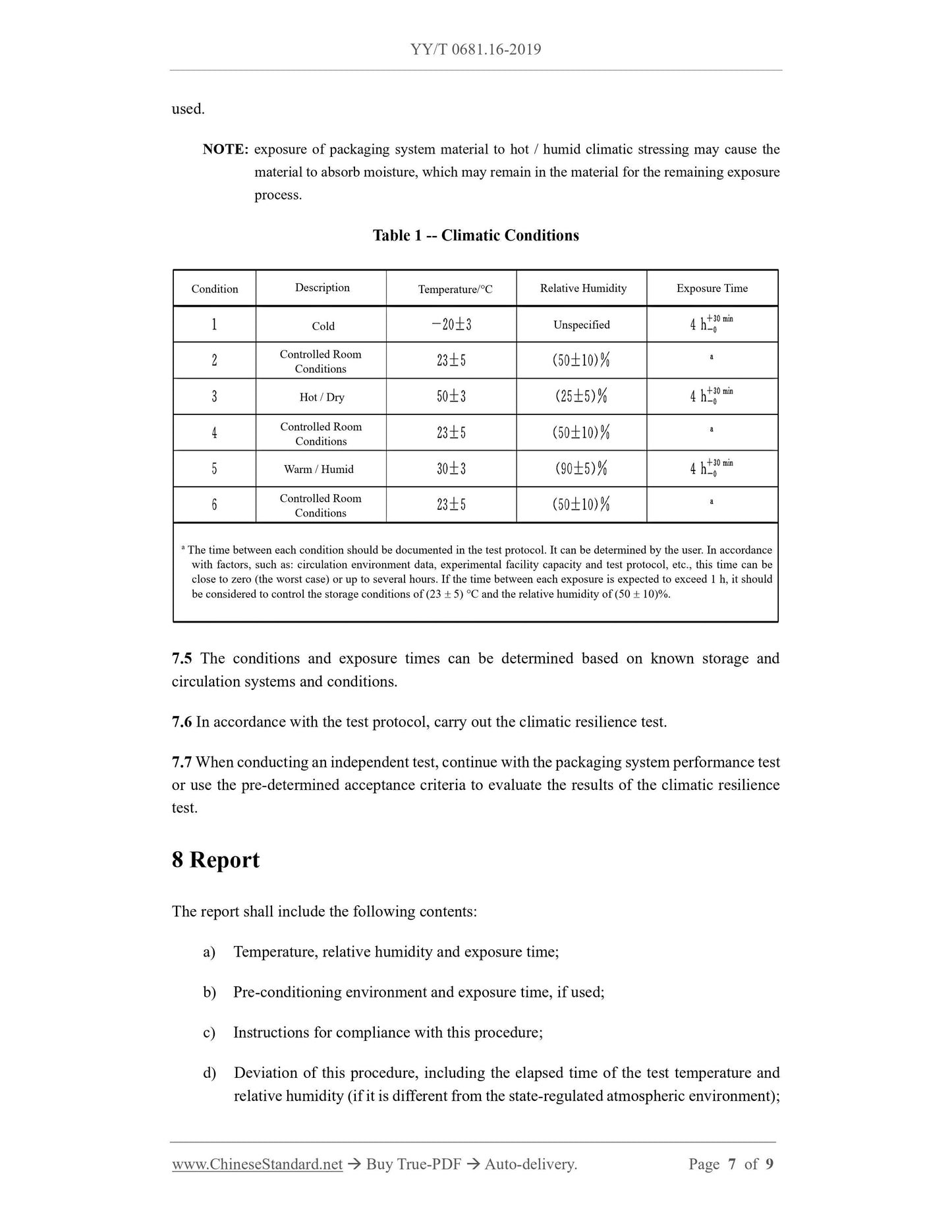
Table 1 Climatic conditions
7.5 The conditions and exposure time can be determined based on known storage and circulation systems and conditions.
7.6 Conduct climate resilience tests according to the test plan.
7.7 When conducting independent tests, continue to perform packaging system performance tests or use pre-determined acceptance criteria to evaluate climate resilience tests
the result of.
8 reports
The report should include the following.
a) Temperature, relative humidity and exposure time;
b) Pre-condition adjustment environment and exposure time, if used;
d) Deviations from this procedure, including the elapsed time of test temperature and relative humidity (if different from the state-conditioned atmospheric environment);
e) When used as an independent test, whether it meets the acceptance criteria and the nature of non-conformity.
Get Quotation: Click YY/T 0681.16-2019 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 0681.16-2019
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 0681.16-2019: Test methods for sterile medical device package - Part 16: Test for climatic stressing of packaging system
YY/T 0681.16-2019
Test methods for sterile medical device package-Part 16.Test for climatic stressing of packaging system
ICS 11.080.040
C31
People's Republic of China Pharmaceutical Industry Standard
Sterile medical device packaging test methods
Part 16.Climate Resilience Test of Packaging System
2019-05-31 released
2020-06-01 Implementation
Issued by the State Drug Administration
Preface
YY/T 0681 "Test Methods for Sterile Medical Device Packaging" consists of the following parts.
---Part 1.Guide to accelerated aging test;
---Part 2.Sealing strength of soft barrier materials;
---Part 3.Unconstrained packaging resists internal pressure damage;
---Part 4.Dyeing liquid penetration method to determine the seal leakage of breathable packaging;
---Part 5.Internal pressure method to detect gross leaks (bubble method);
---Part 6.Evaluation of chemical resistance of printing ink and coating on flexible packaging materials;
---Part 7.Use tape to evaluate the adhesion of ink or coating on flexible packaging materials;
---Part 8.Determination of the weight of the adhesive layer;
---Part 9.Seal burst test of flexible packaging by air pressure inside restraint plate;
---Part 10.Microbial barrier grading test for breathable packaging materials;
---Part 11.Visually inspect the seal integrity of medical packaging;
---Part 12.Anti-rubbing of soft barrier film;
---Part 13.Soft barrier film and composite film resistance to slow puncture;
---Part 14.Test of moisture and dry microbial barrier of breathable packaging materials;
---Part 15.Performance test of transport containers and systems;
---Part 16.Test of Climate Response Ability of Packaging System.
This part is part 16 of YY/T 0681.
This section was drafted in accordance with the rules given in GB/T 1.1-2009.
Please note that certain contents of this document may involve patents. The issuing agency of this document is not responsible for identifying these patents.
This part was proposed by the State Drug Administration.
This part is under the jurisdiction of the National Standardization Technical Committee for Medical Infusion Devices (SAC/TC106).
The main drafting units of this section. Shandong Medical Device Product Quality Inspection Center, Shandong Weigao Group Medical Polymer Products Co., Ltd.
Company, Shanghai MicroPort Medical Equipment (Group) Co., Ltd.
The main drafters of this section. Wang Dongwei, Li Weiyang, Li Yong, Dong Dandan.
Sterile medical device packaging test methods
Part 16.Climate Resilience Test of Packaging System
1 Scope
This part of YY/T 0681 specifies the climatic stress that the sterile medical device packaging system may bear during the circulation cycle in the laboratory.
It can still provide a unified benchmark for product protection from damage or change under the effect of change.
This part is applicable as an independent test to evaluate the climate resilience of the packaging system; it is also applicable to the packaging system as a single package.
The system is subjected to the state adjustment method before the test required for overnight or two-day supply.
This section does not apply to the climate resilience test of refrigerated, frozen or low-temperature storage and transportation packaging systems.
2 Terms and definitions
The following terms and definitions apply to this document.
2.1
Climate strain
Expose the test sample of the packaging system to temperature and humidity conditions for a specified period of time to simulate the expected storage and
Circulation system conditions.
3 overview
Devices that are transported in the circulation environment (regardless of the mode of transport) will be transported to various places (especially import and export) at various times during the year.
Various climates and physical environments. This part is designed to establish the climatic conditions and duration that may be encountered during transportation to the packaging system.
The climate change caused by the system provides guidance to evaluate the ability of packaging to protect the contents or devices during the circulation process.
4 Significance and application
This section provides methods for adjusting the state of the packaging system using the climatic conditions that occur in the actual circulation process. Recommended exposure
The level is based on information about existing transportation, handling and storage environments, current industrial regulations, and published research. These conditions
Not absolute extreme conditions, but they are the average of the daily records of cold and hot climates in the world.
5 Instruments
5.1 The room (or box), whose specifications enable a single sample container or package to be exposed to circulating air at the selected temperature and humidity.
5.2 The control instrument can maintain the indoor environment within the allowable limits of the required environmental conditions (see Table 1).
5.3 Hygrometer, an instrument used to indicate relative humidity, its relative humidity accuracy should be ±2%. The wet and dry bulb thermometer can directly measure the relative
Humidity can also be used to verify hygrometer.
5.4 Thermometers and temperature measuring equipment with an accuracy of 0.1℃ can be used. It can be directly measured with a dry bulb thermometer or a wet bulb thermometer
Or check the temperature indicator.
6 Sample
6.1 The sample should contain a representative packaging system, including the actual contents or devices. Can use defective or less defective interior
The premise is that the defect is not in the research scope and the defect is recorded in the report. Simulated contents can also be used, provided they are
The loading characteristics of the contents are the same.
6.2 Care should be taken to ensure that the packaging system and its contents do not degrade before being exposed to weather stress. This is for the need to send samples
It is especially important when going to a remote test site.
7 Procedure
7.1 Describe the packaging system according to specifications, weight and composition materials, and determine the test unit (shipping box, multiple packages, pallet goods, etc.).
7.2 Identify the various transportation modes and durations used for storage and the circulation of products to customers. For example, products will be shipped by land
And air freight is sent to the destination after a two-day shipping period.
7.3 When used in an independent test, the acceptance criteria are determined in advance.
7.4 Use the sequence, conditions and exposure time given in Table 1 to write a test plan. If the conditions and exposure of the user’s circulation environment are known
If the time is different from that specified in Table 1, the user’s circulation conditions should be used.
Note. Exposing packaging system materials to hot/humid climate strains may cause the materials to absorb moisture, which may remain in the remaining exposure process
In the material.
Table 1 Climatic conditions
7.5 The conditions and exposure time can be determined based on known storage and circulation systems and conditions.
7.6 Conduct climate resilience tests according to the test plan.
7.7 When conducting independent tests, continue to perform packaging system performance tests or use pre-determined acceptance criteria to evaluate climate resilience tests
the result of.
8 reports
The report should include the following.
a) Temperature, relative humidity and exposure time;
b) Pre-condition adjustment environment and exposure time, if used;
d) Deviations from this procedure, including the elapsed time of test temperature and relative humidity (if different from the state-conditioned atmospheric environment);
e) When used as an independent test, whether it meets the acceptance criteria and the nature of non-conformity.
Share