1
/
of
12
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 0758-2021 English PDF (YY/T0758-2021)
YY/T 0758-2021 English PDF (YY/T0758-2021)
Regular price
$230.00 USD
Regular price
Sale price
$230.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY/T 0758-2021 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 0758-2021
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 0758-2021: General requirements for medical laser fiber
YY/T 0758-2021
General requirements for medical laser fiber
ICS 11.404
C40
People's Republic of China Pharmaceutical Industry Standard
Replacing YY/T 0758-2009
General requirements for medical laser fibers
Published on 2021-09-06
2022-09-01 Implementation
Released by the State Drug Administration
directory
Preface I
1 Scope 1
2 Normative references 1
3 Terms and Definitions 1
4 Structural composition and classification 2
5 Requirements 2
6 Test method 5
7 Packaging, marking, instruction manual, transportation and storage 9
General requirements for medical laser fibers
1 Scope
This standard specifies general requirements for medical laser fibers.
This standard applies to medical laser optical fibers (hereinafter referred to as optical fibers) that transmit laser light.
This standard does not apply to optical fibers with gain function.
If the optical fiber forms part of the equipment and cannot be removed from the equipment, the equipment must comply with the relevant national standards (such as GB 9706.1,
GB 9706.20, GB 7247.1, etc.), and refer to this standard. However, if the fiber can be removed from the equipment, the removed unit should
Complies with the applicable requirements of this standard.
2 Normative references
The following documents are essential for the application of this document. For dated references, only the dated version applies to this article
pieces. For undated references, the latest edition (including all amendments) applies to this document.
GB 9706.1 Medical Electrical Equipment Part 1.General Requirements for Basic Safety and Essential Performance
GB 9706.19 Medical Electrical Equipment Part 2.Special Requirements for Safety of Endoscopic Equipment
GB/T 14233.1-2008 Test methods for medical infusion, blood transfusion and injection equipment - Part 1.Chemical analysis methods
GB/T 16886.1 Biological Evaluation of Medical Devices Part 1.Evaluation and Testing in Risk Management Process
YY/T 0313 Requirements for medical polymer product packaging and information provided by manufacturers
YY 9706.102 Medical Electrical Equipment Part 1-2.General Requirements for Basic Safety and Essential Performance Collateral Standard. Electromagnetic and
capacity requirements and tests
ISO 11146 (all parts) Test methods for laser beam width, divergence angle and beam transmission ratio for lasers and laser-related equipment
Pharmacopoeia of the People's Republic of China 2020 Edition
3 Terms and Definitions
The following terms and definitions apply to this document.
3.1
medical laser fiber medicallaserfiber
A product that uses optical fiber as the propagation medium to transmit laser energy.
3.2
Fiber application end fiberapplicator
An applied part formed directly at the end of an optical fiber transmission body.
3.3
External application side additionalapplicator
Applied parts connected to the end of the fiber optic transmission body.
Note. Such as handpieces, microcontrollers, etc.
3.4
Optical fiber transmission body opticalfibercable
The part of the optical fiber used to transmit the laser is generally composed of the core, the cladding, and the coating.
Note. The coating layer includes buffer layer and protective layer.
3.5
connector
A device that connects an optical fiber to a laser device.
3.6
tensile strength
The maximum tensile force that the material can withstand.
4 Structural composition and classification
4.1 Structural composition
Medical laser fiber is mainly composed of connector, fiber transmission body, application end and protective cap.
4.2 Product Classification
Classification by use. Medical laser fiber can be divided into treatment, lighting, inspection and diagnosis.
Classification by application end type. Medical laser fiber can be divided into fiber application end and external application end; among them, the fiber application end is classified according to the fiber head end
Structural classification. It can be divided into flush-cut fiber and non-flummed-cut fiber, among which non-flush-cut fiber includes cylindrical, conical, spherical and hemispherical, microlens light
Fiber, etc.
Classification by number of uses. Medical laser fibers can be divided into disposable and reusable fibers.
5 Requirements
5.1 Product information to be provided by the manufacturer
The manufacturer shall provide at least the following product information.
a) overall length;
b) Core diameter (or fiber bundle diameter);
c) the wavelength (or spectral range) for which the fiber is applicable;
d) minimum transmission efficiency;
e) maximum transmission power (or energy);
f) details of cleaning, disinfection or sterilization methods;
g) tensile strength;
h) Minimum bending working radius of optical fiber;
i) head end structure type (if applicable);
j) connector type;
k) Numerical aperture.
5.2 Dimensions
5.2.1 Overall length
The manufacturer shall give the nominal value and tolerance of the total length of the optical fiber, and the tolerance shall not exceed ±20%.
5.2.2 Core diameter (or fiber bundle diameter)
The manufacturer shall give the nominal value and tolerance of the core diameter (or fiber bundle diameter), and the tolerance shall not exceed ±10%. If the fiber input and the output
If the core diameter (or fiber bundle diameter) of the outgoing end is inconsistent, the nominal value and tolerance should be given respectively, and the tolerance should not exceed ±10%.
5.3 Optical properties
5.3.1 Maximum transmission power (or energy) of optical fiber
The manufacturer shall give the nominal value of the maximum transmission power (or energy) of the fiber during the specified working time when the fiber transmits the specified wavelength.
The value should not be less than the nominal value.
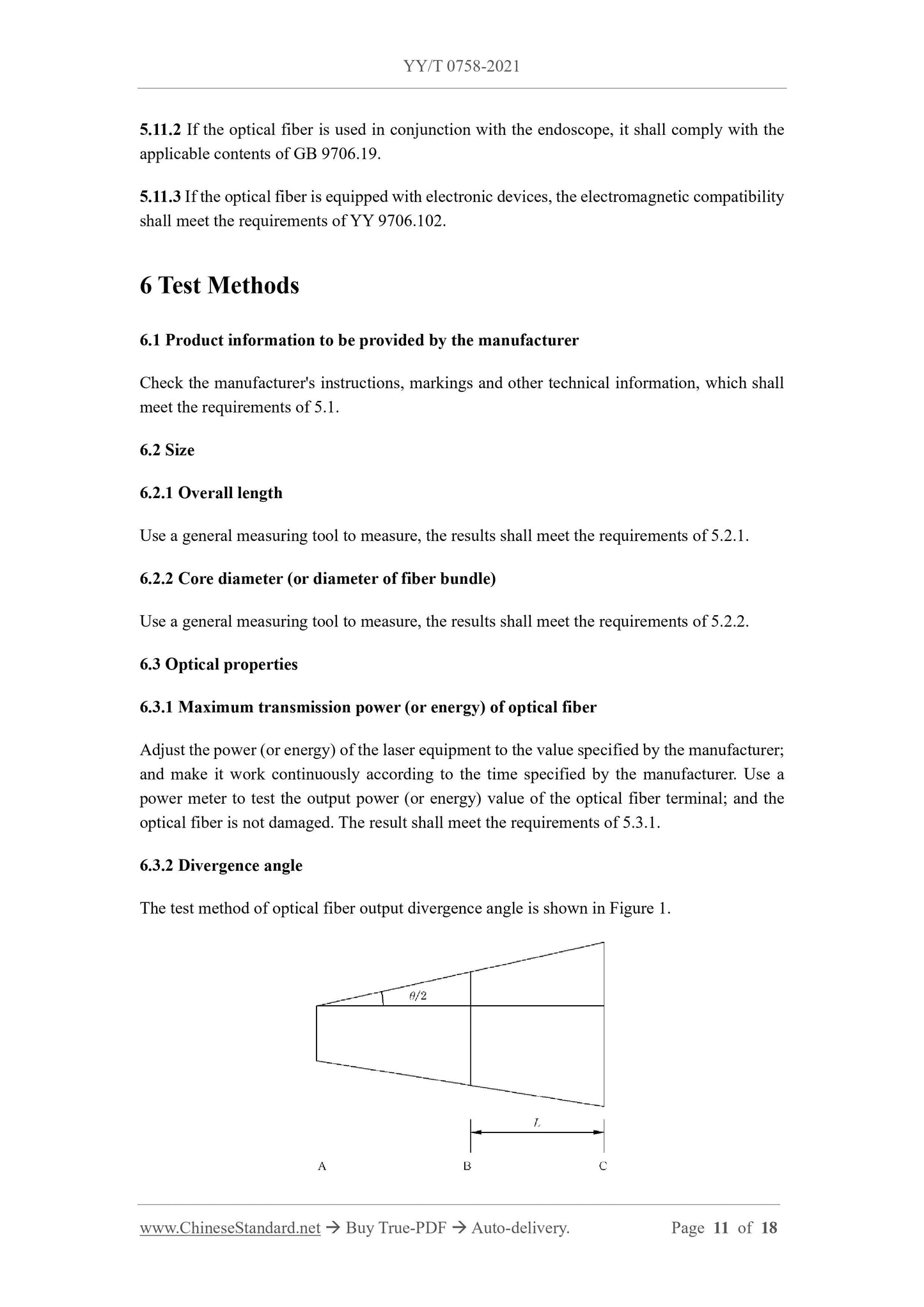
5.3.2 Divergence angle
If the application end of the optical fiber is a flat-cut optical fiber, the manufacturer shall provide the nominal value (or range) and tolerance of the output divergence angle of the optical fiber terminal, and the tolerance shall not exceed
over ±20%.
5.3.3 Optical fiber transmission efficiency
5.3.3.1 When the optical fiber is placed straight, the transmission efficiency of the corresponding wavelength should not be less than the value specified by the manufacturer.
5.3.3.2 The transmission efficiency of the reusable optical fiber can still reach the manufacturer's specified value after the insertion and removal test.
5.3.4 Sterilization
Disposable non-sterile optical fibers and reusable optical fibers shall be sterilized and sterilized according to the methods specified in the instructions. After the test, the optical fibers shall be transmitted
Efficiency can still reach the manufacturer's specified value.
5.4 Mechanical properties
5.4.1 Fiber Tensile Strength
The tensile strength of the joint between the optical fiber transmission body and the connector, and the joint between the optical fiber transmission body and the application end should not be less than the manufacturer's specified value.
After the tensile test, the fiber should be intact without damage.
5.4.2 Minimum bending radius of optical fiber
If the fiber is allowed to be bent, the manufacturer shall specify the minimum bending working radius. When bent to this value, the transmission efficiency of the fiber shall not be reduced.
Less than 90% when placed flat.
5.4.3 Fiber bending fatigue resistance
Unless otherwise specified, the fiber shall be capable of being repeatedly bent 100 times at the minimum bending working radius of the fiber provided by the manufacturer. bending
After the anti-fatigue test, the optical fiber transmission efficiency can still reach the manufacturer's specified value.
5.5 Requirements for non-flush-cut fibers
5.5.1 Cylinder fiber
5.5.1.1 The manufacturer shall give the luminous length, outer diameter and tolerance of the cylinder, and the tolerance shall not exceed ±20%.
5.5.1.2 The manufacturer shall give the maximum bending angle of the light-emitting cylinder and the fiber body. After the bending test, there is no crack between the light-emitting cylinder and the fiber body.
The transmission power shall meet the requirements of 5.3.1.
5.5.1.3 The uniformity of the power density distribution of the cylindrical fiber shall not be greater than ±20%.
5.5.1.4 The manufacturer shall give the proportion of light emitted from the end face.
5.5.2 Spherical (or hemispherical) fibers
5.5.2.1 The manufacturer shall give the outer diameter and tolerance of the spherical (or hemispherical) end, and the tolerance shall not exceed ±20%.
5.5.2.2 If the output is focused after passing through a spherical (or hemispherical) fiber, the manufacturer shall provide the focal spot size, focal length and tolerance, the tolerance shall not exceed
±20%.
5.5.2.3 If the output is uniform after passing through a spherical (or hemispherical) optical fiber, the uniformity of the spot power density distribution should not be greater than ±20%.
5.5.3 Microlens Fiber
5.5.3.1 The manufacturer shall give the outer diameter and tolerance of the microlens fiber, and the tolerance shall not exceed ±20%.
5.5.3.2 The uniformity of the spot power density distribution should not be greater than ±20%.
5.5.4 Oblique-firing fibers
5.5.4.1 The manufacturer shall give the angle and tolerance between the oblique beam and the optical fiber transmission body, and the tolerance shall not exceed ±20%.
5.5.4.2 The manufacturer shall give the terminal output divergence angle (or the spot size at the working distance) and the tolerance, and the tolerance shall not exceed ±20%.
5.6 Requirements for external applications
5.6.1 Light needle
5.6.1.1 The manufacturer shall give the length, outer diameter and tolerance of the optical needle, and the tolerance shall not exceed ±20%.
5.6.1.2 If the optical needle can be bent, the bending angle and tolerance shall be given, and the tolerance shall not exceed ±20%.
5.6.1.3 The manufacturer shall give the nominal value and tolerance of the terminal output divergence angle, and the tolerance shall not exceed ±20%.
5.6.2 Hand tools
5.6.2.1 The manufacturer shall give the diameter and tolerance of the hand tool, and the tolerance shall not exceed ±20%.
5.6.2.2 The manufacturer shall give the transmission efficiency of the tools used to connect the optical fibers.
5.6.2.3 Carry out the disinfection and sterilization test of the external hand tool according to the method specified in the instruction manual. After the test, the transmission efficiency of the external hand tool can still reach the manufacturing
manufacturer's specified value.
5.7 Appearance
The surface of the fiber should be smooth and free of sharp edges, burrs and cracks. The parts are connected compactly and are not easy to peel off.
5.8 Sterility
Fibers packaged in sterile packaging should be sterile.
5.9 Residual Amount of Ethylene Oxide
If the optical fiber is sterilized with ethylene oxide, the residual amount of ethylene oxide in the optical fiber should not be greater than 0.1 mg/piece.
5.10 Biocompatibility
Parts of equipment parts and accessories that are expected to come into contact with biological tissues, cells or body fluids should follow the guidelines given in GB/T 16886.1
and principles are evaluated and demonstrated.
5.11 Safety performance
5.11.1 Electrical safety shall meet the requirements of GB 9706.1.
Get Quotation: Click YY/T 0758-2021 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 0758-2021
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 0758-2021: General requirements for medical laser fiber
YY/T 0758-2021
General requirements for medical laser fiber
ICS 11.404
C40
People's Republic of China Pharmaceutical Industry Standard
Replacing YY/T 0758-2009
General requirements for medical laser fibers
Published on 2021-09-06
2022-09-01 Implementation
Released by the State Drug Administration
directory
Preface I
1 Scope 1
2 Normative references 1
3 Terms and Definitions 1
4 Structural composition and classification 2
5 Requirements 2
6 Test method 5
7 Packaging, marking, instruction manual, transportation and storage 9
General requirements for medical laser fibers
1 Scope
This standard specifies general requirements for medical laser fibers.
This standard applies to medical laser optical fibers (hereinafter referred to as optical fibers) that transmit laser light.
This standard does not apply to optical fibers with gain function.
If the optical fiber forms part of the equipment and cannot be removed from the equipment, the equipment must comply with the relevant national standards (such as GB 9706.1,
GB 9706.20, GB 7247.1, etc.), and refer to this standard. However, if the fiber can be removed from the equipment, the removed unit should
Complies with the applicable requirements of this standard.
2 Normative references
The following documents are essential for the application of this document. For dated references, only the dated version applies to this article
pieces. For undated references, the latest edition (including all amendments) applies to this document.
GB 9706.1 Medical Electrical Equipment Part 1.General Requirements for Basic Safety and Essential Performance
GB 9706.19 Medical Electrical Equipment Part 2.Special Requirements for Safety of Endoscopic Equipment
GB/T 14233.1-2008 Test methods for medical infusion, blood transfusion and injection equipment - Part 1.Chemical analysis methods
GB/T 16886.1 Biological Evaluation of Medical Devices Part 1.Evaluation and Testing in Risk Management Process
YY/T 0313 Requirements for medical polymer product packaging and information provided by manufacturers
YY 9706.102 Medical Electrical Equipment Part 1-2.General Requirements for Basic Safety and Essential Performance Collateral Standard. Electromagnetic and
capacity requirements and tests
ISO 11146 (all parts) Test methods for laser beam width, divergence angle and beam transmission ratio for lasers and laser-related equipment
Pharmacopoeia of the People's Republic of China 2020 Edition
3 Terms and Definitions
The following terms and definitions apply to this document.
3.1
medical laser fiber medicallaserfiber
A product that uses optical fiber as the propagation medium to transmit laser energy.
3.2
Fiber application end fiberapplicator
An applied part formed directly at the end of an optical fiber transmission body.
3.3
External application side additionalapplicator
Applied parts connected to the end of the fiber optic transmission body.
Note. Such as handpieces, microcontrollers, etc.
3.4
Optical fiber transmission body opticalfibercable
The part of the optical fiber used to transmit the laser is generally composed of the core, the cladding, and the coating.
Note. The coating layer includes buffer layer and protective layer.
3.5
connector
A device that connects an optical fiber to a laser device.
3.6
tensile strength
The maximum tensile force that the material can withstand.
4 Structural composition and classification
4.1 Structural composition
Medical laser fiber is mainly composed of connector, fiber transmission body, application end and protective cap.
4.2 Product Classification
Classification by use. Medical laser fiber can be divided into treatment, lighting, inspection and diagnosis.
Classification by application end type. Medical laser fiber can be divided into fiber application end and external application end; among them, the fiber application end is classified according to the fiber head end
Structural classification. It can be divided into flush-cut fiber and non-flummed-cut fiber, among which non-flush-cut fiber includes cylindrical, conical, spherical and hemispherical, microlens light
Fiber, etc.
Classification by number of uses. Medical laser fibers can be divided into disposable and reusable fibers.
5 Requirements
5.1 Product information to be provided by the manufacturer
The manufacturer shall provide at least the following product information.
a) overall length;
b) Core diameter (or fiber bundle diameter);
c) the wavelength (or spectral range) for which the fiber is applicable;
d) minimum transmission efficiency;
e) maximum transmission power (or energy);
f) details of cleaning, disinfection or sterilization methods;
g) tensile strength;
h) Minimum bending working radius of optical fiber;
i) head end structure type (if applicable);
j) connector type;
k) Numerical aperture.
5.2 Dimensions
5.2.1 Overall length
The manufacturer shall give the nominal value and tolerance of the total length of the optical fiber, and the tolerance shall not exceed ±20%.
5.2.2 Core diameter (or fiber bundle diameter)
The manufacturer shall give the nominal value and tolerance of the core diameter (or fiber bundle diameter), and the tolerance shall not exceed ±10%. If the fiber input and the output
If the core diameter (or fiber bundle diameter) of the outgoing end is inconsistent, the nominal value and tolerance should be given respectively, and the tolerance should not exceed ±10%.
5.3 Optical properties
5.3.1 Maximum transmission power (or energy) of optical fiber
The manufacturer shall give the nominal value of the maximum transmission power (or energy) of the fiber during the specified working time when the fiber transmits the specified wavelength.
The value should not be less than the nominal value.
5.3.2 Divergence angle
If the application end of the optical fiber is a flat-cut optical fiber, the manufacturer shall provide the nominal value (or range) and tolerance of the output divergence angle of the optical fiber terminal, and the tolerance shall not exceed
over ±20%.
5.3.3 Optical fiber transmission efficiency
5.3.3.1 When the optical fiber is placed straight, the transmission efficiency of the corresponding wavelength should not be less than the value specified by the manufacturer.
5.3.3.2 The transmission efficiency of the reusable optical fiber can still reach the manufacturer's specified value after the insertion and removal test.
5.3.4 Sterilization
Disposable non-sterile optical fibers and reusable optical fibers shall be sterilized and sterilized according to the methods specified in the instructions. After the test, the optical fibers shall be transmitted
Efficiency can still reach the manufacturer's specified value.
5.4 Mechanical properties
5.4.1 Fiber Tensile Strength
The tensile strength of the joint between the optical fiber transmission body and the connector, and the joint between the optical fiber transmission body and the application end should not be less than the manufacturer's specified value.
After the tensile test, the fiber should be intact without damage.
5.4.2 Minimum bending radius of optical fiber
If the fiber is allowed to be bent, the manufacturer shall specify the minimum bending working radius. When bent to this value, the transmission efficiency of the fiber shall not be reduced.
Less than 90% when placed flat.
5.4.3 Fiber bending fatigue resistance
Unless otherwise specified, the fiber shall be capable of being repeatedly bent 100 times at the minimum bending working radius of the fiber provided by the manufacturer. bending
After the anti-fatigue test, the optical fiber transmission efficiency can still reach the manufacturer's specified value.
5.5 Requirements for non-flush-cut fibers
5.5.1 Cylinder fiber
5.5.1.1 The manufacturer shall give the luminous length, outer diameter and tolerance of the cylinder, and the tolerance shall not exceed ±20%.
5.5.1.2 The manufacturer shall give the maximum bending angle of the light-emitting cylinder and the fiber body. After the bending test, there is no crack between the light-emitting cylinder and the fiber body.
The transmission power shall meet the requirements of 5.3.1.
5.5.1.3 The uniformity of the power density distribution of the cylindrical fiber shall not be greater than ±20%.
5.5.1.4 The manufacturer shall give the proportion of light emitted from the end face.
5.5.2 Spherical (or hemispherical) fibers
5.5.2.1 The manufacturer shall give the outer diameter and tolerance of the spherical (or hemispherical) end, and the tolerance shall not exceed ±20%.
5.5.2.2 If the output is focused after passing through a spherical (or hemispherical) fiber, the manufacturer shall provide the focal spot size, focal length and tolerance, the tolerance shall not exceed
±20%.
5.5.2.3 If the output is uniform after passing through a spherical (or hemispherical) optical fiber, the uniformity of the spot power density distribution should not be greater than ±20%.
5.5.3 Microlens Fiber
5.5.3.1 The manufacturer shall give the outer diameter and tolerance of the microlens fiber, and the tolerance shall not exceed ±20%.
5.5.3.2 The uniformity of the spot power density distribution should not be greater than ±20%.
5.5.4 Oblique-firing fibers
5.5.4.1 The manufacturer shall give the angle and tolerance between the oblique beam and the optical fiber transmission body, and the tolerance shall not exceed ±20%.
5.5.4.2 The manufacturer shall give the terminal output divergence angle (or the spot size at the working distance) and the tolerance, and the tolerance shall not exceed ±20%.
5.6 Requirements for external applications
5.6.1 Light needle
5.6.1.1 The manufacturer shall give the length, outer diameter and tolerance of the optical needle, and the tolerance shall not exceed ±20%.
5.6.1.2 If the optical needle can be bent, the bending angle and tolerance shall be given, and the tolerance shall not exceed ±20%.
5.6.1.3 The manufacturer shall give the nominal value and tolerance of the terminal output divergence angle, and the tolerance shall not exceed ±20%.
5.6.2 Hand tools
5.6.2.1 The manufacturer shall give the diameter and tolerance of the hand tool, and the tolerance shall not exceed ±20%.
5.6.2.2 The manufacturer shall give the transmission efficiency of the tools used to connect the optical fibers.
5.6.2.3 Carry out the disinfection and sterilization test of the external hand tool according to the method specified in the instruction manual. After the test, the transmission efficiency of the external hand tool can still reach the manufacturing
manufacturer's specified value.
5.7 Appearance
The surface of the fiber should be smooth and free of sharp edges, burrs and cracks. The parts are connected compactly and are not easy to peel off.
5.8 Sterility
Fibers packaged in sterile packaging should be sterile.
5.9 Residual Amount of Ethylene Oxide
If the optical fiber is sterilized with ethylene oxide, the residual amount of ethylene oxide in the optical fiber should not be greater than 0.1 mg/piece.
5.10 Biocompatibility
Parts of equipment parts and accessories that are expected to come into contact with biological tissues, cells or body fluids should follow the guidelines given in GB/T 16886.1
and principles are evaluated and demonstrated.
5.11 Safety performance
5.11.1 Electrical safety shall meet the requirements of GB 9706.1.
Share