1
/
of
10
PayPal, credit cards. Download editable-PDF & invoice in 1 second!
YY/T 0806-2010 English PDF (YY/T0806-2010)
YY/T 0806-2010 English PDF (YY/T0806-2010)
Regular price
$190.00 USD
Regular price
Sale price
$190.00 USD
Unit price
/
per
Shipping calculated at checkout.
Couldn't load pickup availability
Delivery: 3 seconds. Download true-PDF + Invoice.
Get Quotation: Click YY/T 0806-2010 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 0806-2010
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 0806-2010: Polycarbonate material for manufacture of infusion, transfusion and injection equipments for medical use and other medical devices
YY/T 0806-2010
Polycarbonate material for manufacture of infusion, transfusion and injection equipments for medical use and other medical devices
ICS 11.040.20
C31
People's Republic of China pharmaceutical industry standards
Infusion, transfusion, injections and other medical equipment
Polycarbonate Compound
Polycarbonatematerialformanufactureofinfusion, transfusionandinjection
Issued on. 2010-12-27
2012-06-01 implementation
State Food and Drug Administration issued
Foreword
This standard was drafted in accordance with GB/T 1.1-2009 given rules.
This standard reference ASTMF997-98a (2003) "Medical polycarbonate standards", with non-equivalent.
This standard by the National Standardization Technical Committee Infusion equipment for medical and centralized.
This standard was drafted. Shandong Medical Devices Product Quality Inspection Center.
The main drafters of this standard. Dickson Wong, Wang Ronghui, the first Pan Hua, Guo Lun.
introduction
Polycarbonate (PC) due to the ratio of polyethylene, polystyrene and ABS and other materials with superior physical properties, and have a good outside
Concept, therefore, increasingly applied to the medical infusion, transfusion, injection and other medical equipment, because the material may have a high degree of transparency,
With this material helps achieve full liquid infusion apparatus transparent way.
Infusion, transfusion, injections and other medical equipment
Polycarbonate Compound
1 Scope
This standard specifies the medical infusion, transfusion, injection and other medical equipment with the requirements of special polycarbonate.
This standard does not apply to a polycarbonate copolymer.
2 Normative references
The following documents for the application of this document is essential. For dated references, only the dated version suitable for use herein
Member. For undated references, the latest edition (including any amendments) applies to this document.
1033.1-2008 non-plastic foam density measurement GB/T Part 1. Immersion method, liquid pyknometer method and titration method
(ISO 1183-1.2004, IDT)
GB/T 1843-2008 Determination of Izod impact strength of plastics
GB/T 2547-2008 plastic sampling method
Determination of GB/T 3682 thermoplastic melt flow rate and melt volume flow rate
GB/T 9352-2008 plastic compression molding of thermoplastic material samples
GB/T 14233.1-2008 Infusion, transfusion, injection equipment - Part 1. Chemical analysis
GB/T 16886.1 Biological evaluation of medical devices - Part 1. Evaluation and testing
GB/T 2918-1998 plastic sample conditioning and testing of the standard environment
Laboratory use specifications and test methods GB/T 6682 Analysis
HG/T 3862 plastic yellow index test method
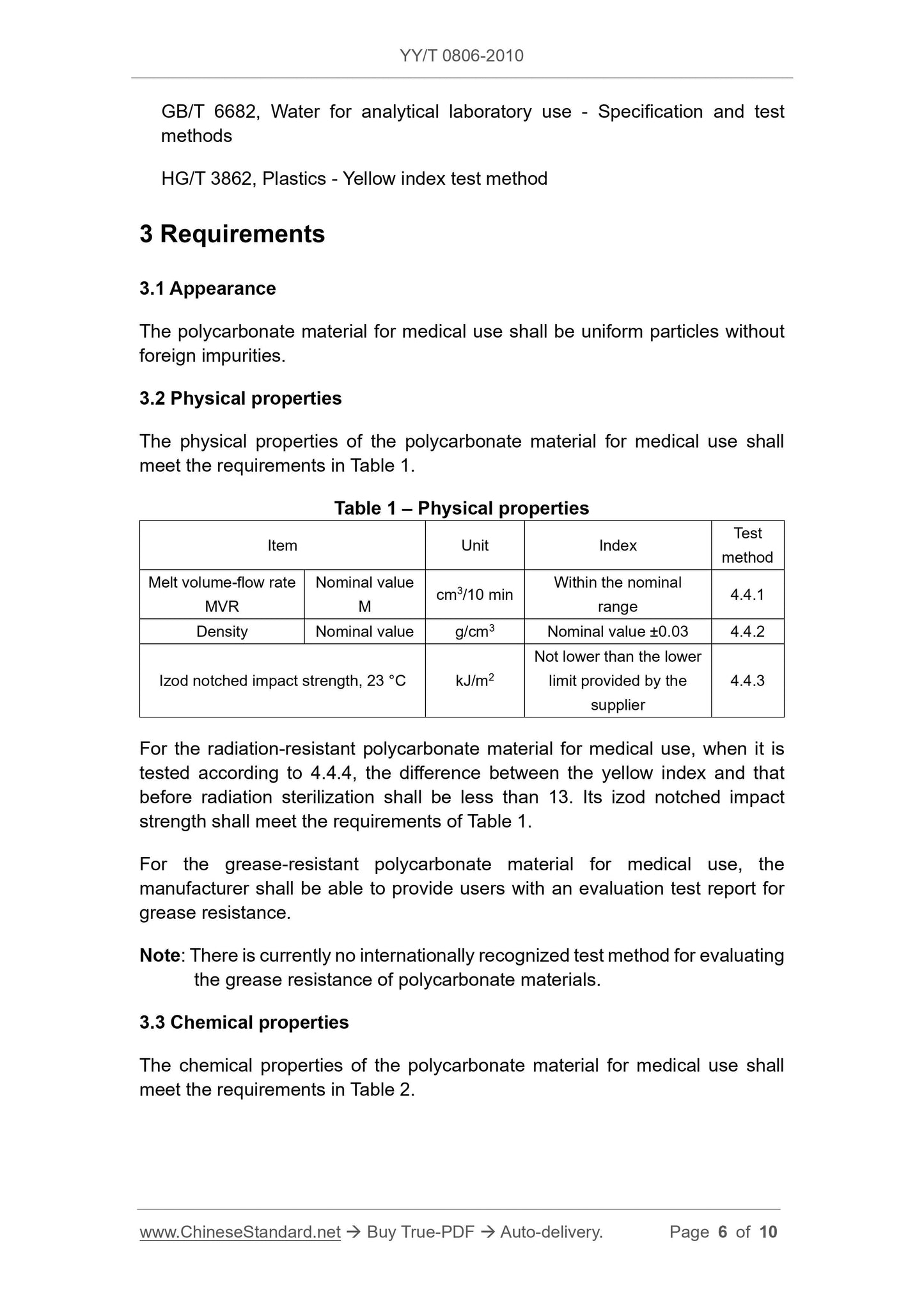
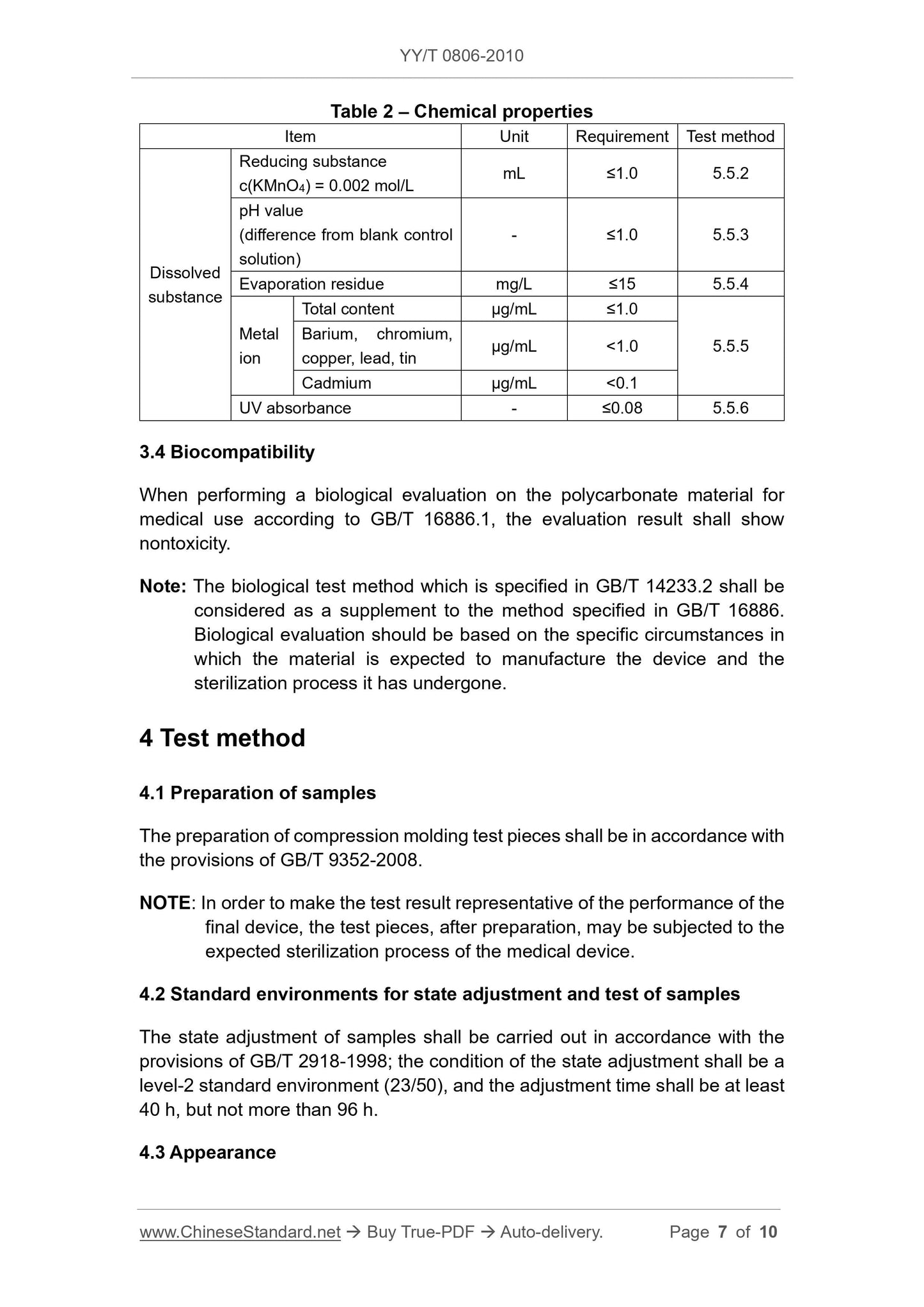
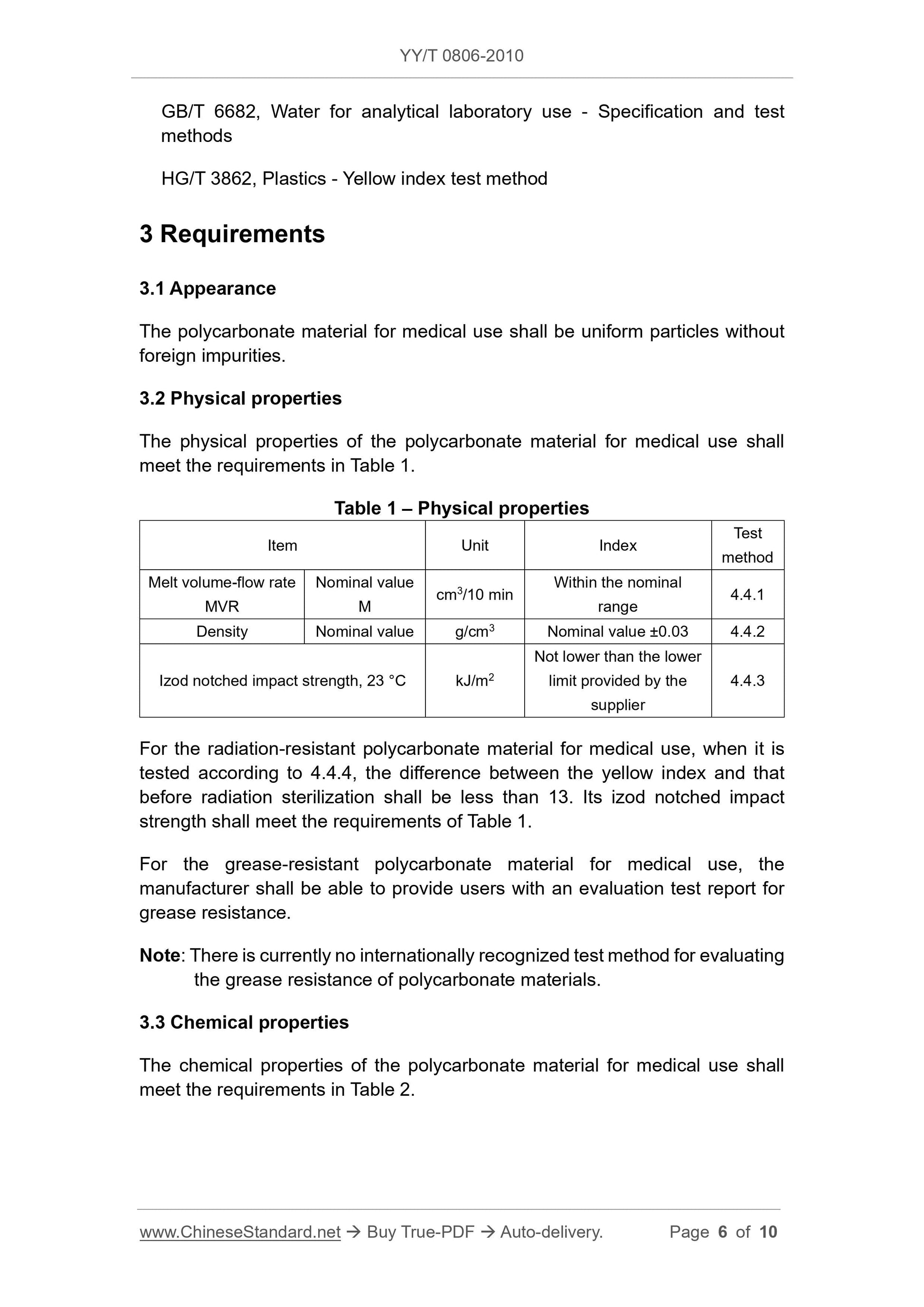
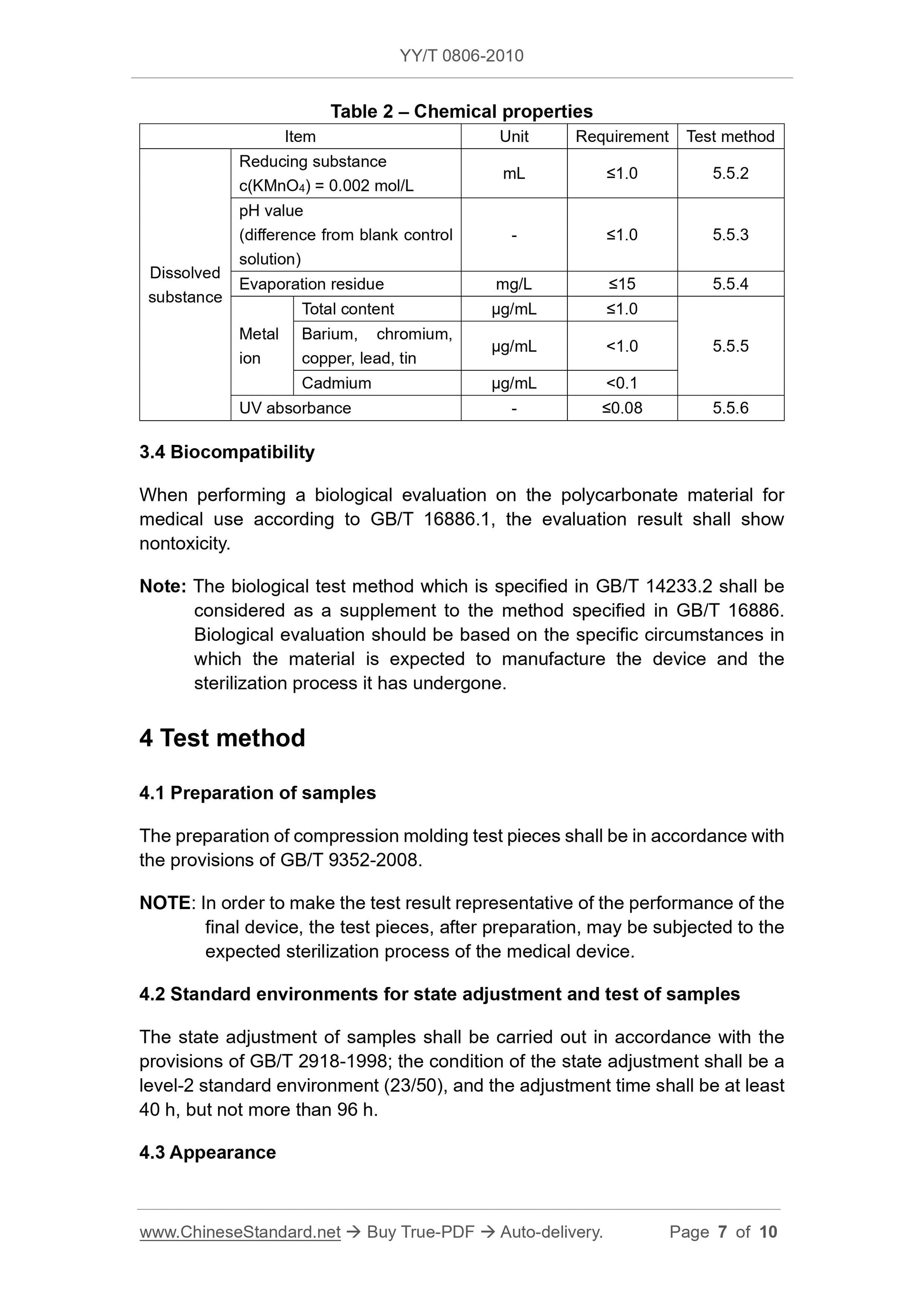
3 Requirements
3.1 Appearance
Medical Compound polycarbonate uniform particles, no foreign impurities.
3.2 Physical properties
The physical properties of the polycarbonate Medical Compound shall comply with the provisions in Table 1.
Table 1 Physical Properties
Item Unit Index Test Method
Melt volume flow rate
MVR
Nominal value
cm3/10min in the nominal range 4.4.1
Nominal Density g/cm3 ± 0.03 4.4.2 nominal value
Izod notched impact strength, 23 ℃ kJ/m2 is not lower than the lower limit provided by the supplier 4.4.3
Get Quotation: Click YY/T 0806-2010 (Self-service in 1-minute)
Historical versions (Master-website): YY/T 0806-2010
Preview True-PDF (Reload/Scroll-down if blank)
YY/T 0806-2010: Polycarbonate material for manufacture of infusion, transfusion and injection equipments for medical use and other medical devices
YY/T 0806-2010
Polycarbonate material for manufacture of infusion, transfusion and injection equipments for medical use and other medical devices
ICS 11.040.20
C31
People's Republic of China pharmaceutical industry standards
Infusion, transfusion, injections and other medical equipment
Polycarbonate Compound
Polycarbonatematerialformanufactureofinfusion, transfusionandinjection
Issued on. 2010-12-27
2012-06-01 implementation
State Food and Drug Administration issued
Foreword
This standard was drafted in accordance with GB/T 1.1-2009 given rules.
This standard reference ASTMF997-98a (2003) "Medical polycarbonate standards", with non-equivalent.
This standard by the National Standardization Technical Committee Infusion equipment for medical and centralized.
This standard was drafted. Shandong Medical Devices Product Quality Inspection Center.
The main drafters of this standard. Dickson Wong, Wang Ronghui, the first Pan Hua, Guo Lun.
introduction
Polycarbonate (PC) due to the ratio of polyethylene, polystyrene and ABS and other materials with superior physical properties, and have a good outside
Concept, therefore, increasingly applied to the medical infusion, transfusion, injection and other medical equipment, because the material may have a high degree of transparency,
With this material helps achieve full liquid infusion apparatus transparent way.
Infusion, transfusion, injections and other medical equipment
Polycarbonate Compound
1 Scope
This standard specifies the medical infusion, transfusion, injection and other medical equipment with the requirements of special polycarbonate.
This standard does not apply to a polycarbonate copolymer.
2 Normative references
The following documents for the application of this document is essential. For dated references, only the dated version suitable for use herein
Member. For undated references, the latest edition (including any amendments) applies to this document.
1033.1-2008 non-plastic foam density measurement GB/T Part 1. Immersion method, liquid pyknometer method and titration method
(ISO 1183-1.2004, IDT)
GB/T 1843-2008 Determination of Izod impact strength of plastics
GB/T 2547-2008 plastic sampling method
Determination of GB/T 3682 thermoplastic melt flow rate and melt volume flow rate
GB/T 9352-2008 plastic compression molding of thermoplastic material samples
GB/T 14233.1-2008 Infusion, transfusion, injection equipment - Part 1. Chemical analysis
GB/T 16886.1 Biological evaluation of medical devices - Part 1. Evaluation and testing
GB/T 2918-1998 plastic sample conditioning and testing of the standard environment
Laboratory use specifications and test methods GB/T 6682 Analysis
HG/T 3862 plastic yellow index test method
3 Requirements
3.1 Appearance
Medical Compound polycarbonate uniform particles, no foreign impurities.
3.2 Physical properties
The physical properties of the polycarbonate Medical Compound shall comply with the provisions in Table 1.
Table 1 Physical Properties
Item Unit Index Test Method
Melt volume flow rate
MVR
Nominal value
cm3/10min in the nominal range 4.4.1
Nominal Density g/cm3 ± 0.03 4.4.2 nominal value
Izod notched impact strength, 23 ℃ kJ/m2 is not lower than the lower limit provided by the supplier 4.4.3
Share